Question
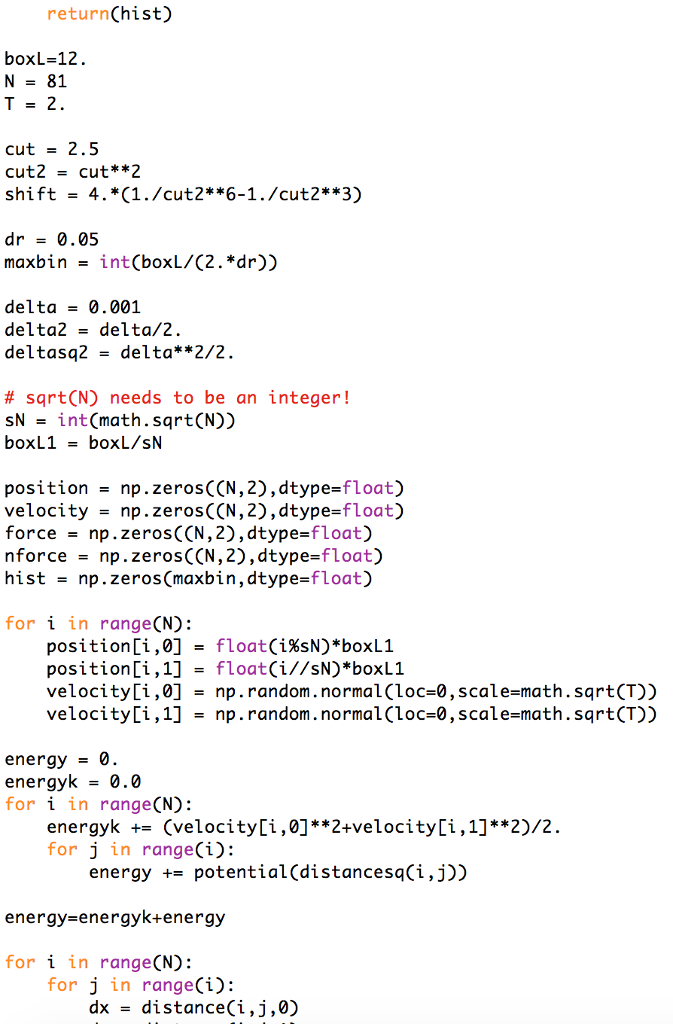
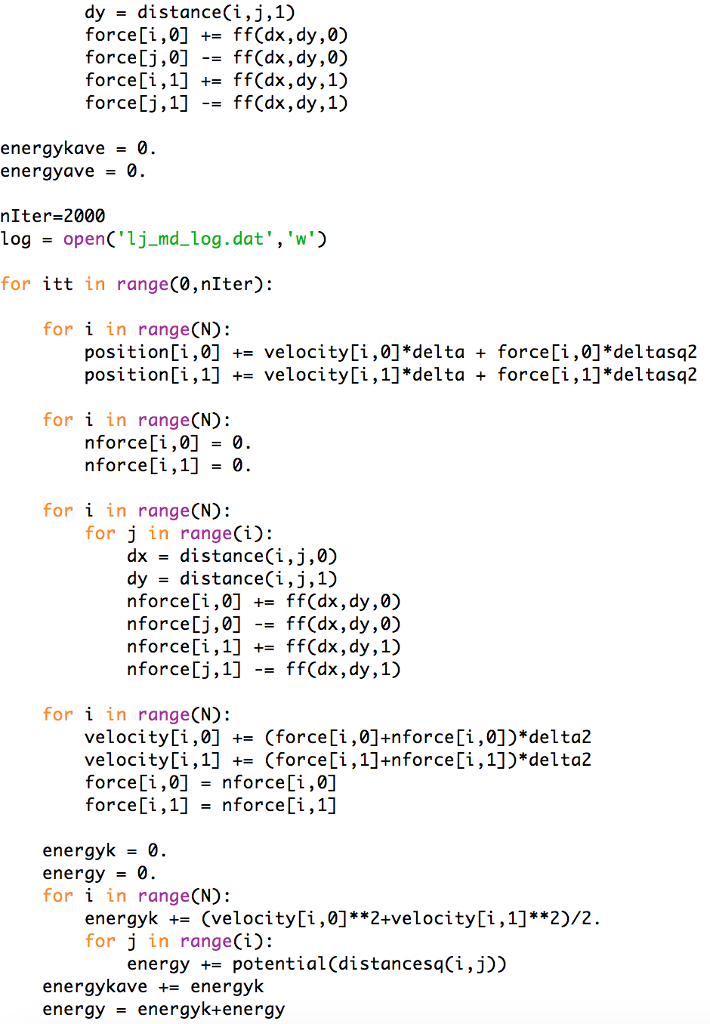
The Lennard-Jone(LJ) Monte Carlo and Molecular Dynamics programs are as the below to calculate average energies (the details depend on the program; for example, the
The Lennard-Jone(LJ) Monte Carlo and Molecular Dynamics programs are as the below to calculate average energies (the details depend on the program; for example, the Monte Carlo code calculates only the average potential energy). Modify one of these programs to calculate the average of the so-called virial,
where v(r) is the interparticle interaction potential. Calculate the average viral for a liquid-like state of the Lennard-Jones system and use it to find it to find the pressure. In d = 2, the pressure and the viral are related in the following way,
where is the number density in d = 2.
You should know your work: I would like to see the relevant part of your code, know the state point conditions that you used, see some evidence of equilibration etc.
Here is the Lennard-Jone(LJ) Monte Carlo and Molecular Dynamics programs

 grout.close()
grout.close()


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


