Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The Lewis structures of four compounds are given. Which of these molecules are polar? Select three. Consider the shape of the molecule. For the molecule
The Lewis structures of four compounds are given.
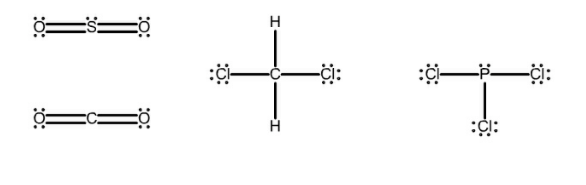
Which of these molecules are polar? Select three. Consider the shape of the molecule. For the molecule to be non-polar the bond polarities need to cancel each other out, often by being directly across from each other.
PCl3
CH2Cl2
SO2
CO2
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


