Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The liquid phase, isotherm, isomerization reaction of glucose to fructose is reversible in the presence of an enzyme according to the following chemical equation: CH,OH
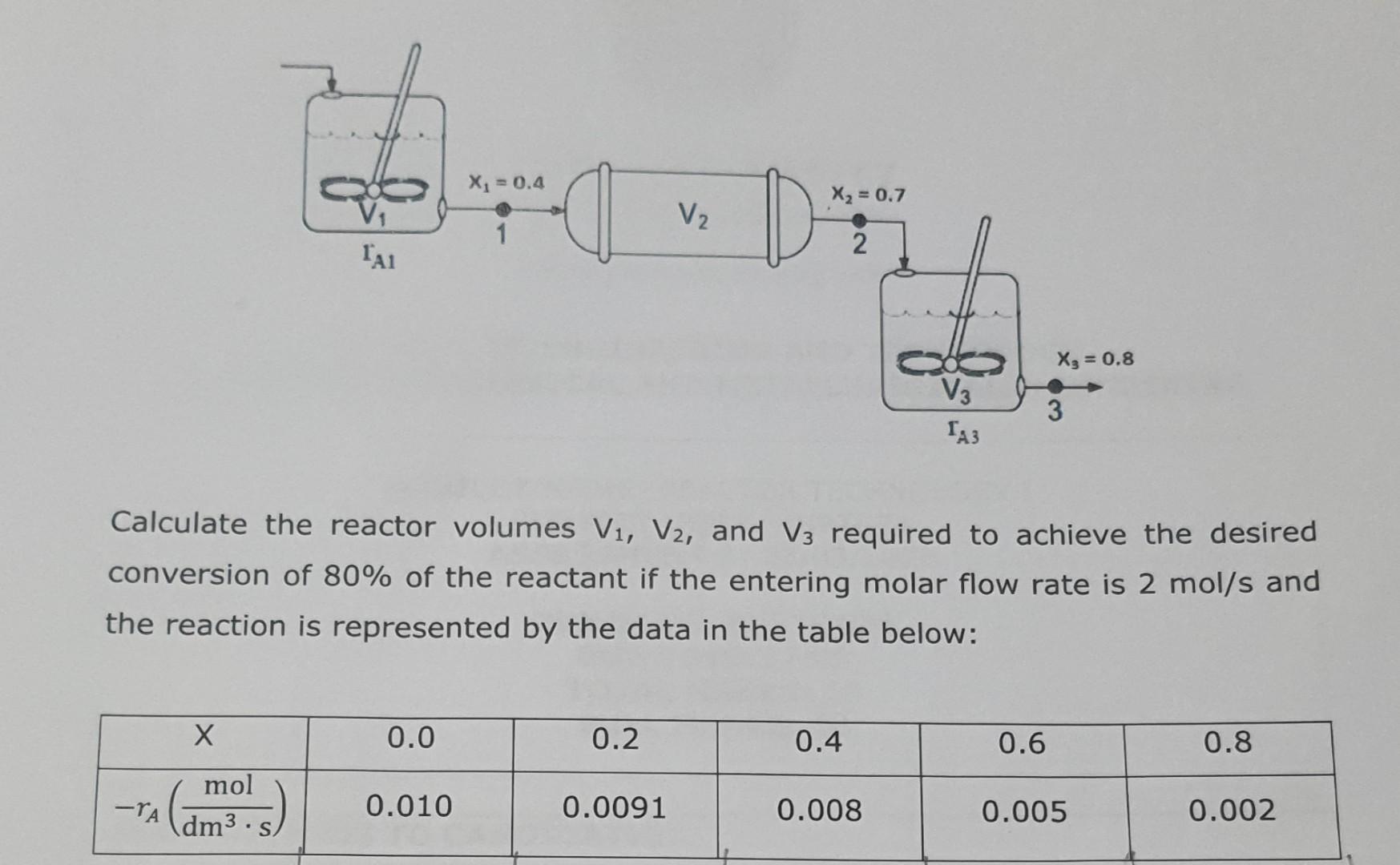
The liquid phase, isotherm, isomerization reaction of glucose to fructose is reversible in the presence of an enzyme according to the following chemical equation: CH,OH HO OH HO OH OH Glucose Isomerase HO OH OH Glucose Fructose The reaction is carried out adiabatically in a series of reactors as per the arrangement below: Xi = 0.4 X2 = 0.7 V2 D 1 1 2 X3 = 0.8 V3 3 TA3 Calculate the reactor volumes V1, V2, and V3 required to achieve the desired conversion of 80% of the reactant if the entering molar flow rate is 2 mol/s and the reaction is represented by the data in the table below: X 0.0 0.2 0.4 0.6 0.8 mol - 0.010 0.0091 0.008 0.005 0.002 dm3 S
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


