Answered step by step
Verified Expert Solution
Question
1 Approved Answer
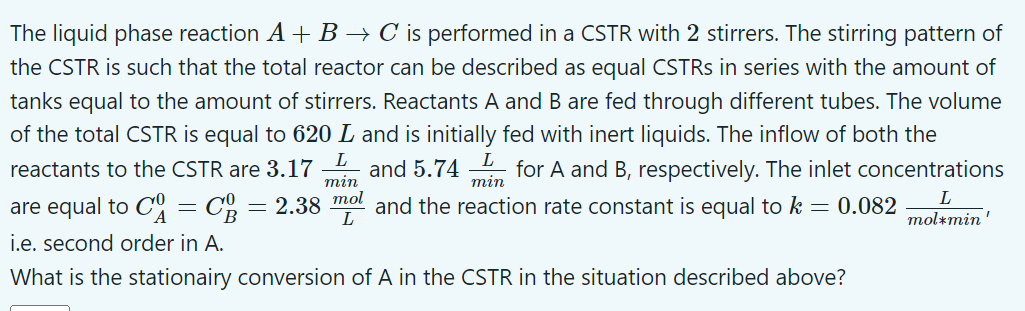
The liquid phase reaction A + B C is performed in a CSTR with 2 stirrers. The stirring pattern of the CSTR is such that
The liquid phase reaction is performed in a CSTR with stirrers. The stirring pattern of
the CSTR is such that the total reactor can be described as equal CSTRs in series with the amount of
tanks equal to the amount of stirrers. Reactants A and are fed through different tubes. The volume
of the total CSTR is equal to and is initially fed with inert liquids. The inflow of both the
reactants to the CSTR are and for A and respectively. The inlet concentrations
are equal to and the reaction rate constant is equal to
ie second order in A
What is the stationairy conversion of in the CSTR in the situation described above?The liquid phase reaction ABC
is performed in a CSTR with
stirrers. The stirring pattern of the CSTR is such that the total reactor can be described as equal CSTRs in series with the amount of tanks equal to the amount of stirrers. Reactants A and B are fed through different tubes. The volume of the total CSTR is equal to
L
and is initially fed with inert liquids. The inflow of both the reactants to the CSTR are
Lmin
and
Lmin
for A and B respectively. The inlet concentrations are equal to CACB
molL
and the reaction rate constant is equal to k
Lmolmin
ie second order in A
What is the stationairy conversion of A in the CSTR in the situation described above?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


