Answered step by step
Verified Expert Solution
Question
1 Approved Answer
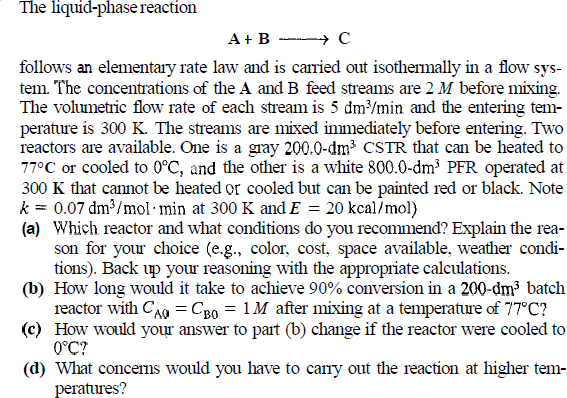
The liquid - phase reaction A + BlongrightarrowC follows an elementary rate law and is carried out isothermally in a flow sys - tem. The
The liquidphase reaction
BlongrightarrowC
follows an elementary rate law and is carried out isothermally in a flow sys
tem. The concentrations of the A and B feed streams are before mixing.
The volumetric flow rate of each stream is and the entering tem
perature is The streams are mixed immediately before entering. Two
reactors are available. One is a gray that can be heated to
or cooled to and the other is a white operated at
that cannot be heated or cooled but can be painted red or black. Note
at and kca
a Which reactor and what conditions do you recommend? Explain the rea
son for your choice eg color, cost, space available, weather condi
tions Back up your reasoning with the appropriate calculations.
b How long would it take to achieve conversion in a batch
reactor with after mixing at a temperature of
c How would your answer to part b change if the reactor were cooled to
d What concerns would you have to carry out the reaction at higher tem
peratures?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


