Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The liquid phase reaction between the methyl bromide and potassium iodide has been found to be first order with respect to each reactant. C2HB12
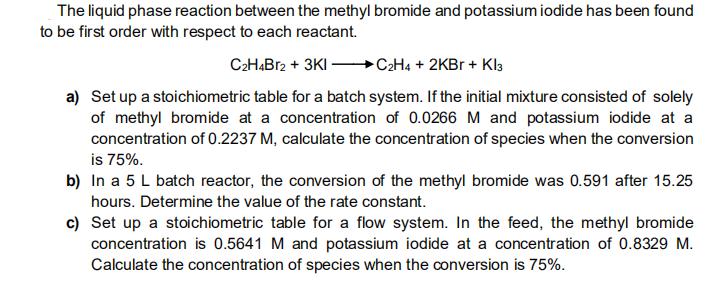
The liquid phase reaction between the methyl bromide and potassium iodide has been found to be first order with respect to each reactant. C2HB12 + 3KI - C2H4 + 2KBR + Kl3 a) Set up a stoichiometric table for a batch system. If the initial mixture consisted of solely of methyl bromide at a concentration of 0.0266 M and potassium iodide at a concentration of 0.2237 M, calculate the concentration of species when the conversion is 75%. b) In a 5 L batch reactor, the conversion of the methyl bromide was 0.591 after 15.25 hours. Determine the value of the rate constant. c) Set up a stoichiometric table for a flow system. In the feed, the methyl bromide concentration is 0.5641 M and potassium iodide at a concentration of 0.8329 M. Calculate the concentration of species when the conversion is 75%.
Step by Step Solution
★★★★★
3.44 Rating (167 Votes )
There are 3 Steps involved in it
Step: 1
a C2H4Br2 initial 00266M conversion 75 so cosnumed Caox 00266075 001995M KI consumed 3001995 00...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


