Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The liquid phase reaction of sodium thiosulfate ( A ) and hydrogen peroxide ( B ) Na , S , 0 , + 2 H
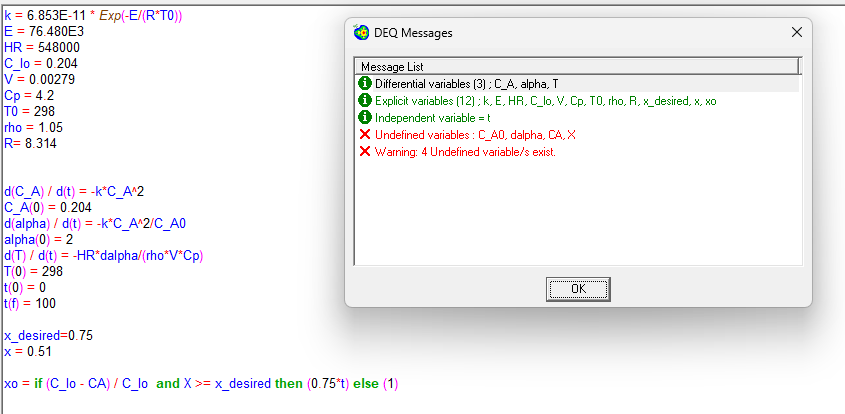
The liquid phase reaction of sodium thiosulfate A and hydrogen peroxide B NaSH Products is carried out in a batch reactor. Assume second order kinetics with respect to A and zero order with respect to B Determine the necessary time and final reaction mixture temperature for conversion under adiabatic conditions. Use the following data. Please correct the code and help me find temp at conversion and time
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


