Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The liquid phase reversible reaction, A+BC+D occurs in a batch reactor at 50C and atmospheric pressure. The forward and reverse reactions are pseudo 1st fast
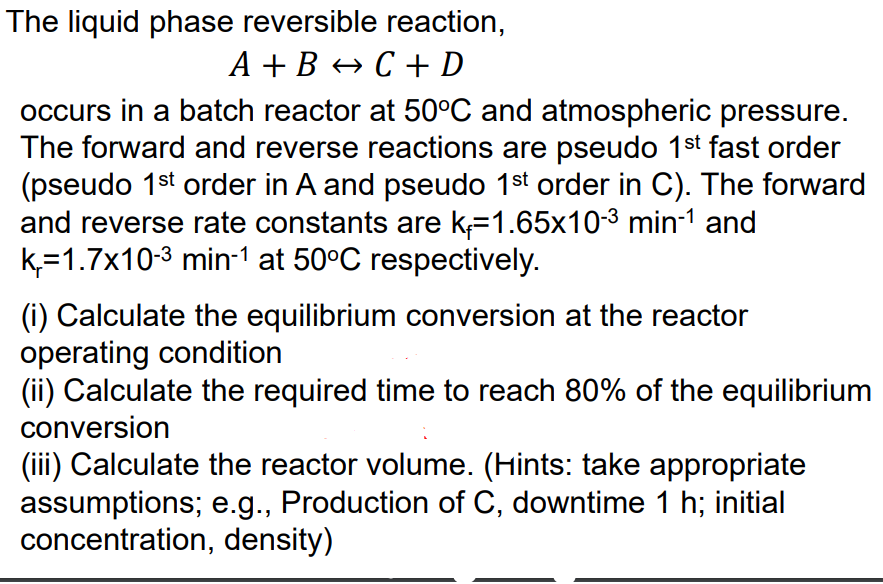 The liquid phase reversible reaction, A+BC+D occurs in a batch reactor at 50C and atmospheric pressure. The forward and reverse reactions are pseudo 1st fast order (pseudo 1st order in A and pseudo 1st order in C). The forward and reverse rate constants are kf=1.65103min1 and kr=1.7103min1 at 50C respectively. (i) Calculate the equilibrium conversion at the reactor operating condition (ii) Calculate the required time to reach 80% of the equilibrium conversion (iii) Calculate the reactor volume. (Hints: take appropriate assumptions; e.g., Production of C, downtime 1h; initial concentration, density) The liquid phase reversible reaction, A+BC+D occurs in a batch reactor at 50C and atmospheric pressure. The forward and reverse reactions are pseudo 1st fast order (pseudo 1st order in A and pseudo 1st order in C). The forward and reverse rate constants are kf=1.65103min1 and kr=1.7103min1 at 50C respectively. (i) Calculate the equilibrium conversion at the reactor operating condition (ii) Calculate the required time to reach 80% of the equilibrium conversion (iii) Calculate the reactor volume. (Hints: take appropriate assumptions; e.g., Production of C, downtime 1h; initial concentration, density)
The liquid phase reversible reaction, A+BC+D occurs in a batch reactor at 50C and atmospheric pressure. The forward and reverse reactions are pseudo 1st fast order (pseudo 1st order in A and pseudo 1st order in C). The forward and reverse rate constants are kf=1.65103min1 and kr=1.7103min1 at 50C respectively. (i) Calculate the equilibrium conversion at the reactor operating condition (ii) Calculate the required time to reach 80% of the equilibrium conversion (iii) Calculate the reactor volume. (Hints: take appropriate assumptions; e.g., Production of C, downtime 1h; initial concentration, density) The liquid phase reversible reaction, A+BC+D occurs in a batch reactor at 50C and atmospheric pressure. The forward and reverse reactions are pseudo 1st fast order (pseudo 1st order in A and pseudo 1st order in C). The forward and reverse rate constants are kf=1.65103min1 and kr=1.7103min1 at 50C respectively. (i) Calculate the equilibrium conversion at the reactor operating condition (ii) Calculate the required time to reach 80% of the equilibrium conversion (iii) Calculate the reactor volume. (Hints: take appropriate assumptions; e.g., Production of C, downtime 1h; initial concentration, density) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


