Question
The liquid-phase reaction follows an elementary rate law and is carried out isothermally in a flow system. The concentrations of the A and B stream
The liquid-phase reaction
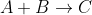
follows an elementary rate law and is carried out isothermally in a flow system. The concentrations of the A and B stream are 2 M before mixing. The volumetric flow rate of each stream is 5 dm3/min, and the entering temperature is 300 K. The streams are mixed immediatly before entering. Two reactors are available. One is a gray 200 dm3 CSTR that can be heated to 77C or cooled to 0C, and the other is a white 800 dm3 PFR operated at 300 K that cannot be heated or cooled but can be painted red or black. Note k=0.07 dm3/mol*min at 300 K and E=20 kcal/mol.
(e) What batch reactor volume would be necessary to process the same amount of species A per day as the flow reactors while achieving 90% conversion? Estimate the costo of the batch reactor.
(f) Write a couple of sentences describing what you learned from the problem and what you believe to be the point of the problem.
A+B+C AStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


