Question
The mass fraction of O2, N2, and CO2 which are mixed inside a rigid tank at 290 K and 150 kPa are 0.32, 0.13,
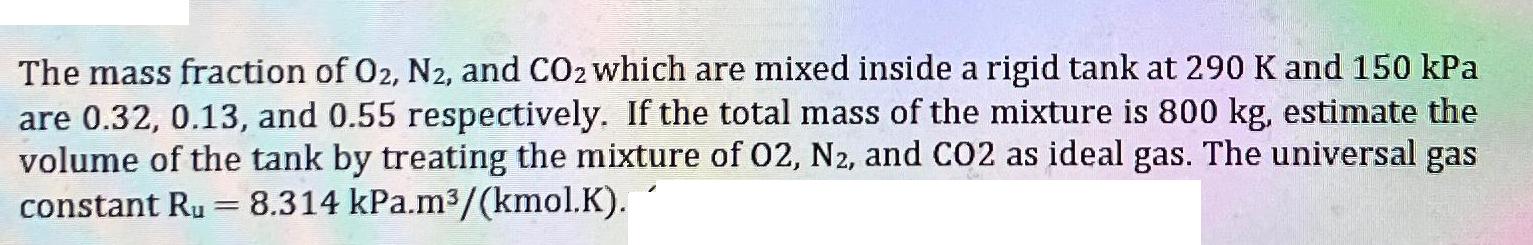
The mass fraction of O2, N2, and CO2 which are mixed inside a rigid tank at 290 K and 150 kPa are 0.32, 0.13, and 0.55 respectively. If the total mass of the mixture is 800 kg, estimate the volume of the tank by treating the mixture of 02, N2, and CO2 as ideal gas. The universal gas constant Ru 8.314 kPa.m/(kmol.K). =
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To estimate the volume of the tank with a mixture of O2 N2 and CO2 gases we can use the ideal gas la...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics for Engineers
Authors: Kenneth A. Kroos, Merle C. Potter
1st edition
1133112862, 978-113311286
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



