Question
The mechanical properties of an epoxy can be measured using the configuration shown in the figure below. The epoxy is initially a liquid and allowed
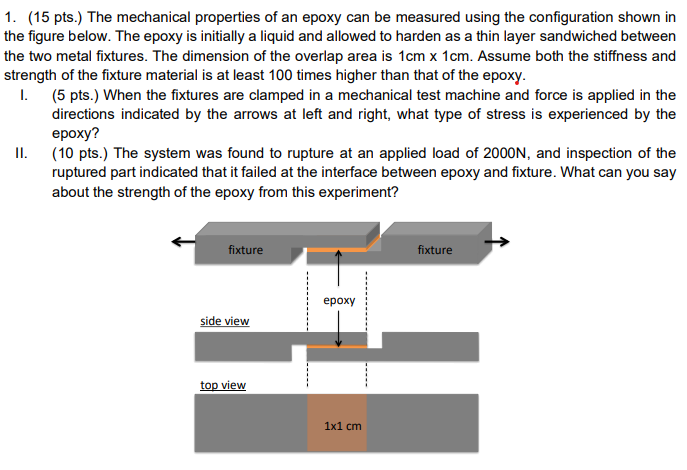
The mechanical properties of an epoxy can be measured using the configuration shown in the figure below. The epoxy is initially a liquid and allowed to harden as a thin layer sandwiched between the two metal fixtures. The dimension of the overlap area is 1cm x 1cm. Assume both the stiffness and strength of the fixture material is at least 100 times higher than that of the epoxy. I. (5 pts.) When the fixtures are clamped in a mechanical test machine and force is applied in the directions indicated by the arrows at left and right, what type of stress is experienced by the epoxy? II. (10 pts.) The system was found to rupture at an applied load of 2000N, and inspection of the ruptured part indicated that it failed at the interface between epoxy and fixture. What can you say about the strength of the epoxy from this experiment?
1. (15 pts.) The mechanical properties of an epoxy can be measured using the configuration shown in the figure below. The epoxy is initially a liquid and allowed to harden as a thin layer sandwiched between the two metal fixtures. The dimension of the overlap area is 1cm x 1cm. Assume both the stiffness and strength of the fixture material is at least 100 times higher than that of the epoxy. 1. (5 pts. When the fixtures are clamped in a mechanical test machine and force is applied in the directions indicated by the arrows at left and right, what type of stress is experienced by the epoxy? II. (10 pts.) The system was found to rupture at an applied load of 2000N, and inspection of the ruptured part indicated that it failed at the interface between epoxy and fixture. What can you say about the strength of the epoxy from this experiment? fixture fixture epoxy side view top view 1x1 cmStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


