Answered step by step
Verified Expert Solution
Question
1 Approved Answer
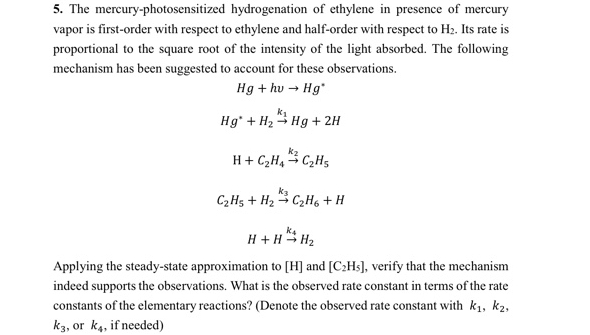
The mercury - photosensitized hydrogenation of ethylene in presence of mercury vapor is first - order with respect to ethylene and half - order with
The mercuryphotosensitized hydrogenation of ethylene in presence of mercury vapor is firstorder with respect to ethylene and halforder with respect to Its rate is proportional to the square root of the intensity of the light absorbed The following mechanism has been suggested to account for these observations.
Applying the steadystate approximation to and verify that the mechanism indeed supports the observations. What is the observed rate constant in terms of the rate constants of the elementary reactions? Denote the observed rate constant with or if needed
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


