Question
The methanol synthesis reaction CO+2H2CH3OH is reversible at typical operating conditions. With certain heterogeneous catalysts, the reaction is thought to proceed according to the
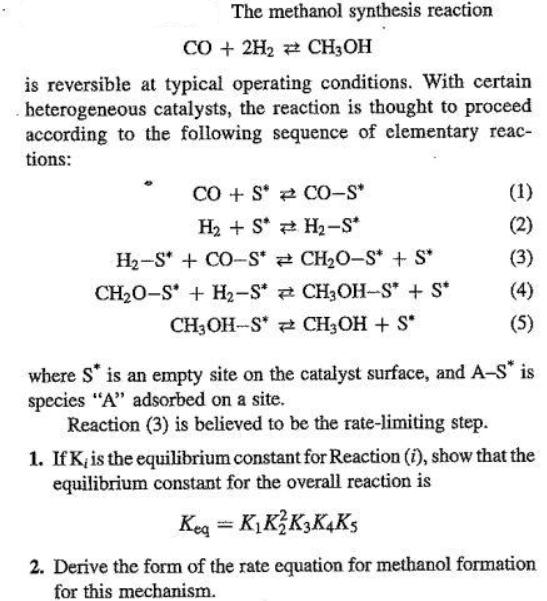
The methanol synthesis reaction CO+2H2CH3OH is reversible at typical operating conditions. With certain heterogeneous catalysts, the reaction is thought to proceed according to the following sequence of elementary reac- tions: COS CO-S* (1) H2S H-S* (2) H2-S +CO-S* CHO-S* + S* (3) CH2O-S+H2-S* CH3OH-S* + S* (4) CH3OH-SCH3OH + S* (5) where S* is an empty site on the catalyst surface, and A-S* is species "A" adsorbed on a site. Reaction (3) is believed to be the rate-limiting step. 1. If K, is the equilibrium constant for Reaction (i), show that the equilibrium constant for the overall reaction is Keq = KKK3K4K5 2. Derive the form of the rate equation for methanol formation for this mechanism.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



