Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The net potential energy between two adjacent ions, EN, may be represented by EN=rA+rnB Where A,B, and n are constants whose values depend on the
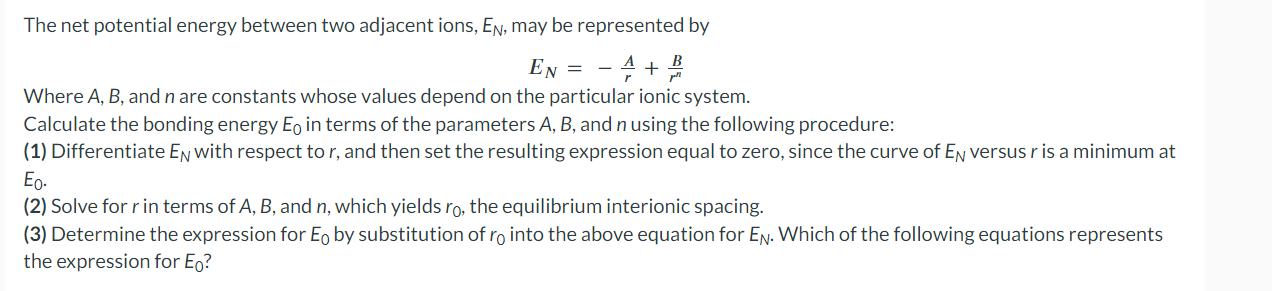 The net potential energy between two adjacent ions, EN, may be represented by EN=rA+rnB Where A,B, and n are constants whose values depend on the particular ionic system. Calculate the bonding energy E0 in terms of the parameters A,B, and n using the following procedure: (1) Differentiate EN with respect to r, and then set the resulting expression equal to zero, since the curve of EN versus r is a minimum at E0 (2) Solve for r in terms of A,B, and n, which yields r0, the equilibrium interionic spacing. (3) Determine the expression for E0 by substitution of r0 into the above equation for EN. Which of the following equations represents the expression for E0
The net potential energy between two adjacent ions, EN, may be represented by EN=rA+rnB Where A,B, and n are constants whose values depend on the particular ionic system. Calculate the bonding energy E0 in terms of the parameters A,B, and n using the following procedure: (1) Differentiate EN with respect to r, and then set the resulting expression equal to zero, since the curve of EN versus r is a minimum at E0 (2) Solve for r in terms of A,B, and n, which yields r0, the equilibrium interionic spacing. (3) Determine the expression for E0 by substitution of r0 into the above equation for EN. Which of the following equations represents the expression for E0 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


