Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The normal boiling point of the benzene-toluene mixture, where the molar fraction of benzene is at 98.6C is 533 mmHg. What is the vapor pressure
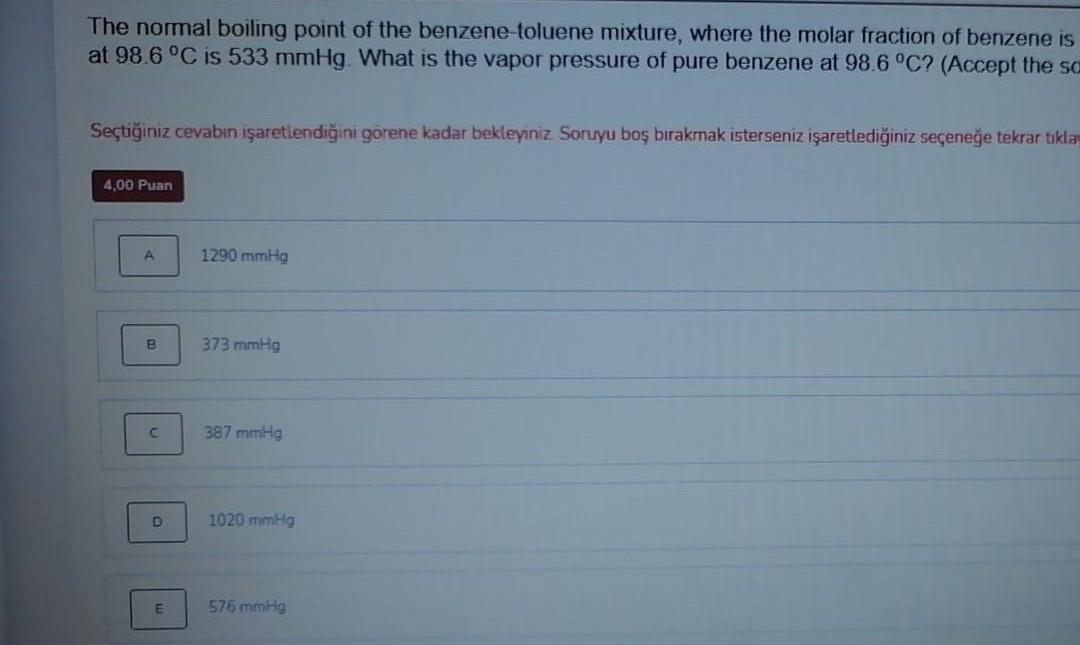
The normal boiling point of the benzene-toluene mixture, where the molar fraction of benzene is at 98.6C is 533 mmHg. What is the vapor pressure of pure benzene at 98.6 C? (Accept the sc Setiiniz cevabn iaretlendiini grene kadar bekleyiniz. Soruyu bo brakmak isterseniz iaretlediiniz seenee tekrar tikla 4,00 Puan A 1290 mmHg B 373 mmHg 387 mmHg D 1020 mmHg E 576 mmHg The normal boiling point of the benzene-toluene mixture, where the molar fraction of benzene is at 98.6C is 533 mmHg. What is the vapor pressure of pure benzene at 98.6 C? (Accept the sc Setiiniz cevabn iaretlendiini grene kadar bekleyiniz. Soruyu bo brakmak isterseniz iaretlediiniz seenee tekrar tikla 4,00 Puan A 1290 mmHg B 373 mmHg 387 mmHg D 1020 mmHg E 576 mmHg
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


