Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 6 On abrading the stainless steel and the aluminium, how do the ranking change in the galvanic series? O aluminium became more cathodic
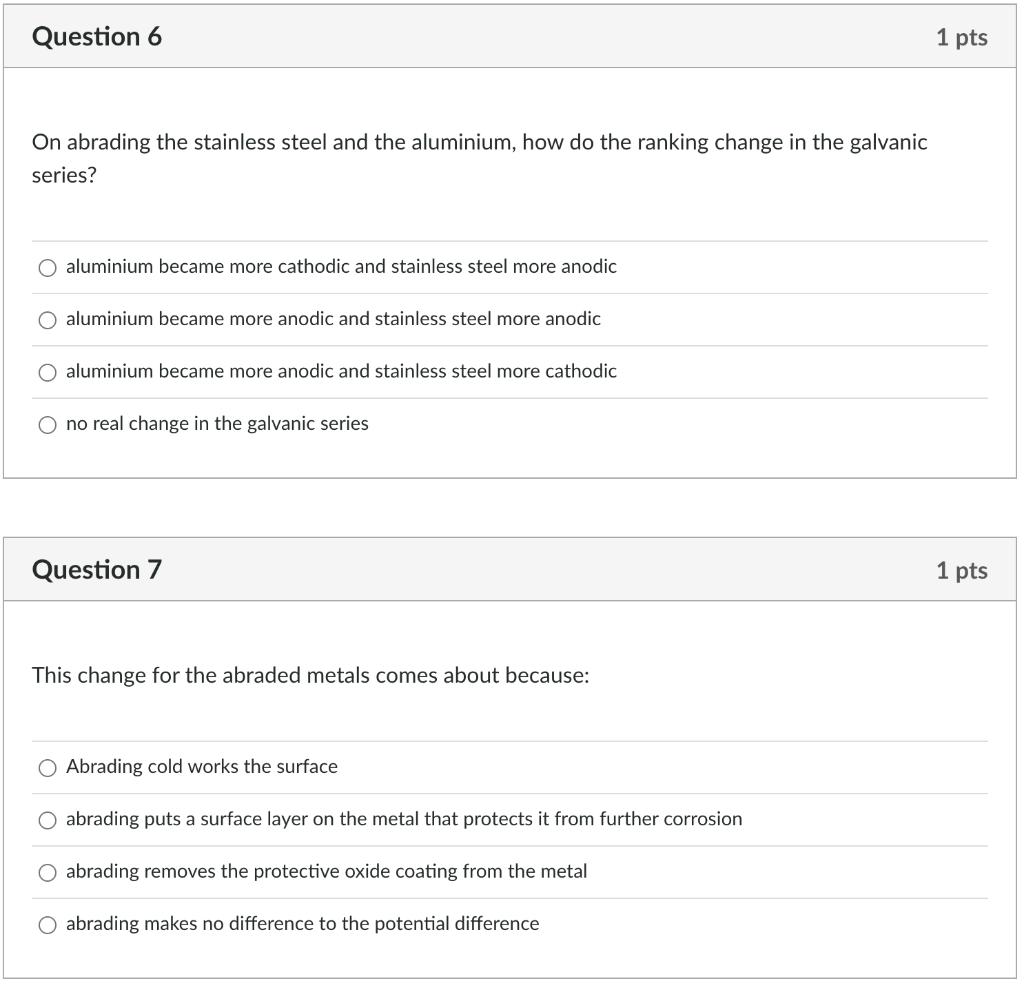
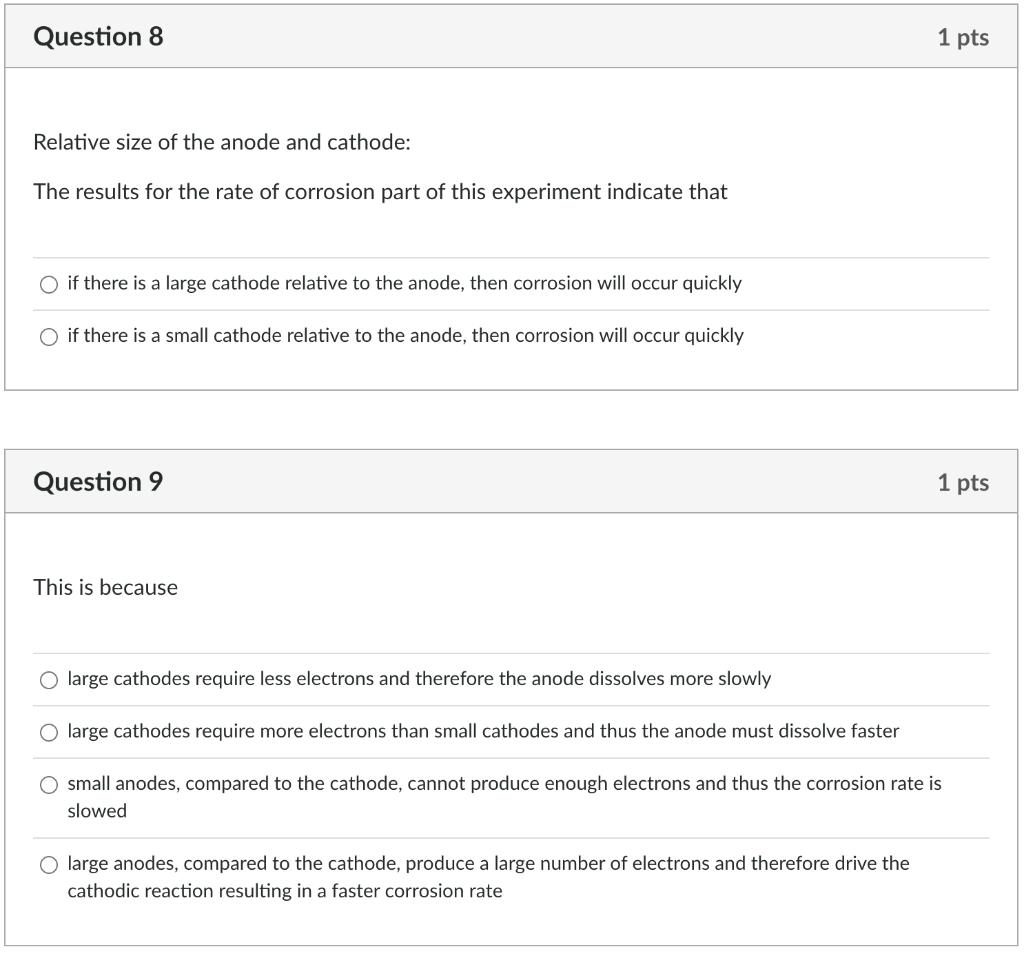
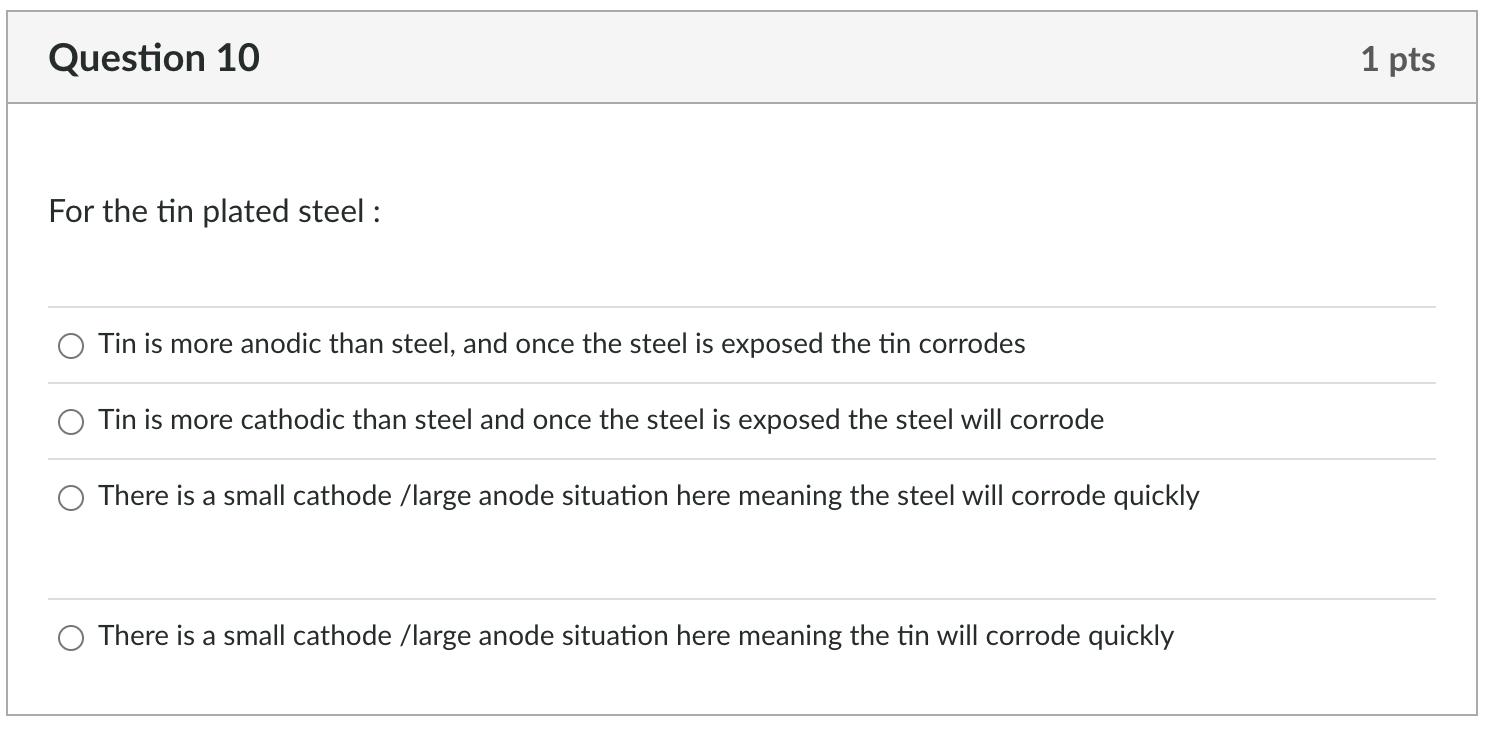
Question 6 On abrading the stainless steel and the aluminium, how do the ranking change in the galvanic series? O aluminium became more cathodic and stainless steel more anodic aluminium became more anodic and stainless steel more anodic O aluminium became more anodic and stainless steel more cathodic no real change in the galvanic series Question 7 This change for the abraded metals comes about because: O Abrading cold works the surface abrading puts a surface layer on the metal that protects it from further corrosion abrading removes the protective oxide coating from the metal O abrading makes no difference to the potential difference 1 pts 1 pts Question 8 Relative size of the anode and cathode: The results for the rate of corrosion part of this experiment indicate that O if there is a large cathode relative to the anode, then corrosion will occur quickly if there is a small cathode relative to the anode, then corrosion will occur quickly Question 9 This is because 1 pts O large anodes, compared to the cathode, produce a large number of electrons and therefore drive the cathodic reaction resulting in a faster corrosion rate 1 pts O large cathodes require less electrons and therefore the anode dissolves more slowly large cathodes require more electrons than small cathodes and thus the anode must dissolve faster small anodes, compared to the cathode, cannot produce enough electrons and thus the corrosion rate is slowed Question 10 For the tin plated steel: Tin is more anodic than steel, and once the steel is exposed the tin corrodes Tin is more cathodic than steel and once the steel is exposed the steel will corrode There is a small cathode /large anode situation here meaning the steel will corrode quickly There is a small cathode /large anode situation here meaning the tin will corrode quickly 1 pts
Step by Step Solution
★★★★★
3.59 Rating (177 Votes )
There are 3 Steps involved in it
Step: 1
Question 6 Answer Aluminum become more anodic and stainless steel become mo...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


