Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The nucleus of an atom consists of protons and neutrons (no electrons). A nucleus of a carbon-12 isotope contains six protons and six neutrons,
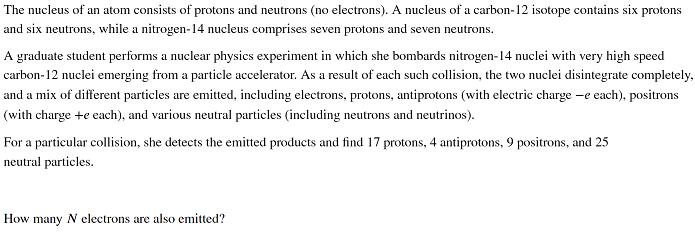
The nucleus of an atom consists of protons and neutrons (no electrons). A nucleus of a carbon-12 isotope contains six protons and six neutrons, while a nitrogen-14 nucleus comprises seven protons and seven neutrons. A graduate student performs a nuclear physics experiment in which she bombards nitrogen-14 nuclei with very high speed carbon-12 nuclei emerging from a particle accelerator. As a result of each such collision, the two nuclei disintegrate completely, and a mix of different particles are emitted, including electrons, protons, antiprotons (with electric charge -e each), positrons (with charge +e each), and various neutral particles (including neutrons and neutrinos). For a particular collision, she detects the emitted products and find 17 protons, 4 antiprotons, 9 positrons, and 25 neutral particles. How many N electrons are also emitted?
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To determine the number of electrons emitted in the particular collision described we start by consi...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


