Answered step by step
Verified Expert Solution
Question
1 Approved Answer
the number i got from the experiment is 1.07 g not sure how to anwer the question. using item (4)-grams of sodium carbonate Na2CO3 on
the number i got from the experiment is 1.07 g not sure how to anwer the question. 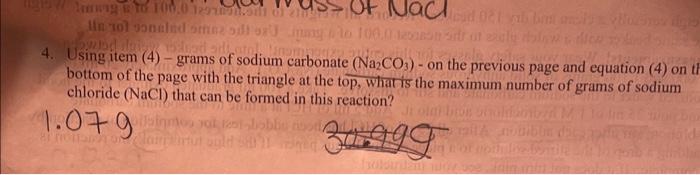
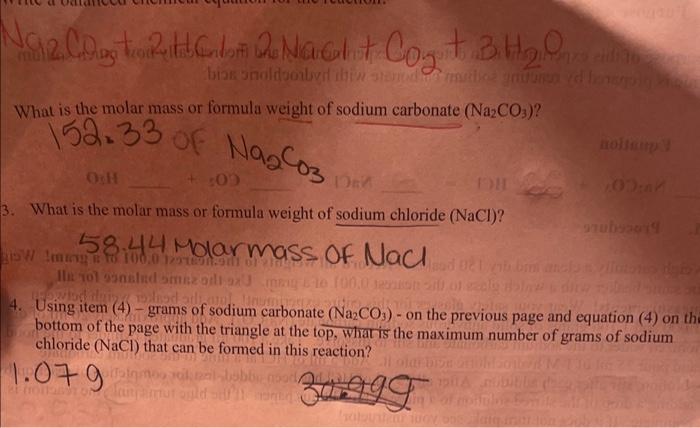
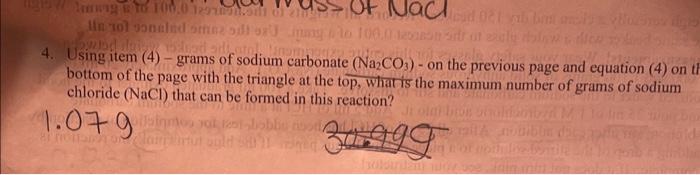
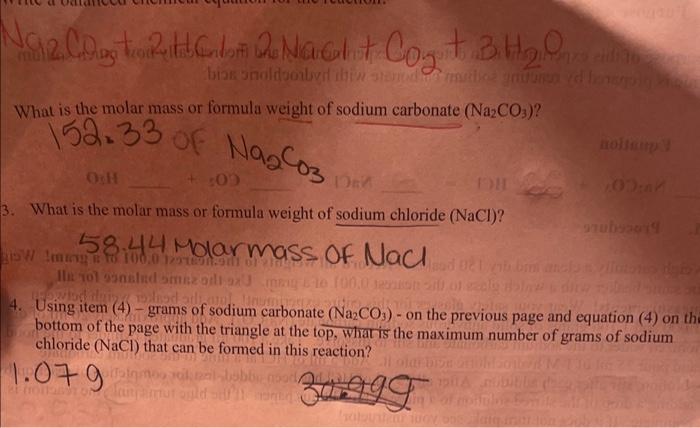
using item (4)-grams of sodium carbonate Na2CO3 on the previous page and equation (4) on thr bottom page with the triangle at the top, what is the maximum number of grams of sodium chloride (NaCl) that can be formed in this reaction. 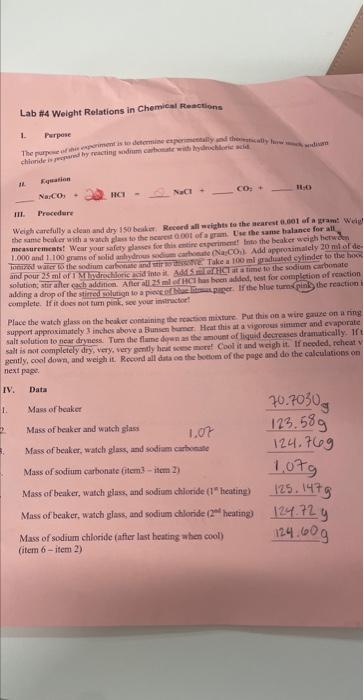

4. Using item (4) - grams of sodium carbonate (Na2CO3) - on the previous page and equation (4) on bottom of the page with the triangle at the top, wharis the maximum number of grams of sodium chloride (NaCl) that can be formed in this reaction? What is the molar mass or formula weight of sodium carbonate (Na2CO3) ? 52330(1Na2CO3 What is the molar mass or formula weight of sodium chloride (NaCl) ? 58.44 molarmass of NaCl 4. Using item (4) - grams of sodium carbonate (Na2CO3) - on the previous page and equation (4) on th bottom of the page with the triangle at the top, whar is the maximum number of grams of sodium chloride (NaCl) that can be formed in this reaction? 1. Parpose 12. Eduation iII. Precedare complete. If it does not tum penk, see your intrictor? Place the watch glass on the hcoker containing the reativa mixtare. Put this on a wire gruze on a ring support approximately 3 inches ahove a Bunsen bamer. Heat elhss at a vigorous siaumer and evaporate salt solution to near dryness. Tum the farne demen as the a mouet of liqual dociesies dramatieally. If sall is not completely dry, very, very gendly heit wese moest Cool it and woigh it. If needed, reheit v geatly, cool down, and weigh it. Reevrd all data ee the betim of the page and do the calculatious on next paget. 1. Write a balanced chemical equation for the reaction! Na2CO3+2HCl=2NaCl+CO2+3H2O 2. What is the molar mass or formula weight of sodium carbonate (Na2CO3) ? 152.33 of Na2CO3 3. What is the molar mass or formula weight of sodium chloride (NaCl) ? 58.44 molarmass of NaCl 4. Using item (4) - grams of sodium carbonate (Na2CO3) - on the previous page and equation (4) on the bottom of the page with the triangle at the top, what is the maximum number of grams of sodium chloride (NaCl) that can be formed in this reaction 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


