Answered step by step
Verified Expert Solution
Question
1 Approved Answer
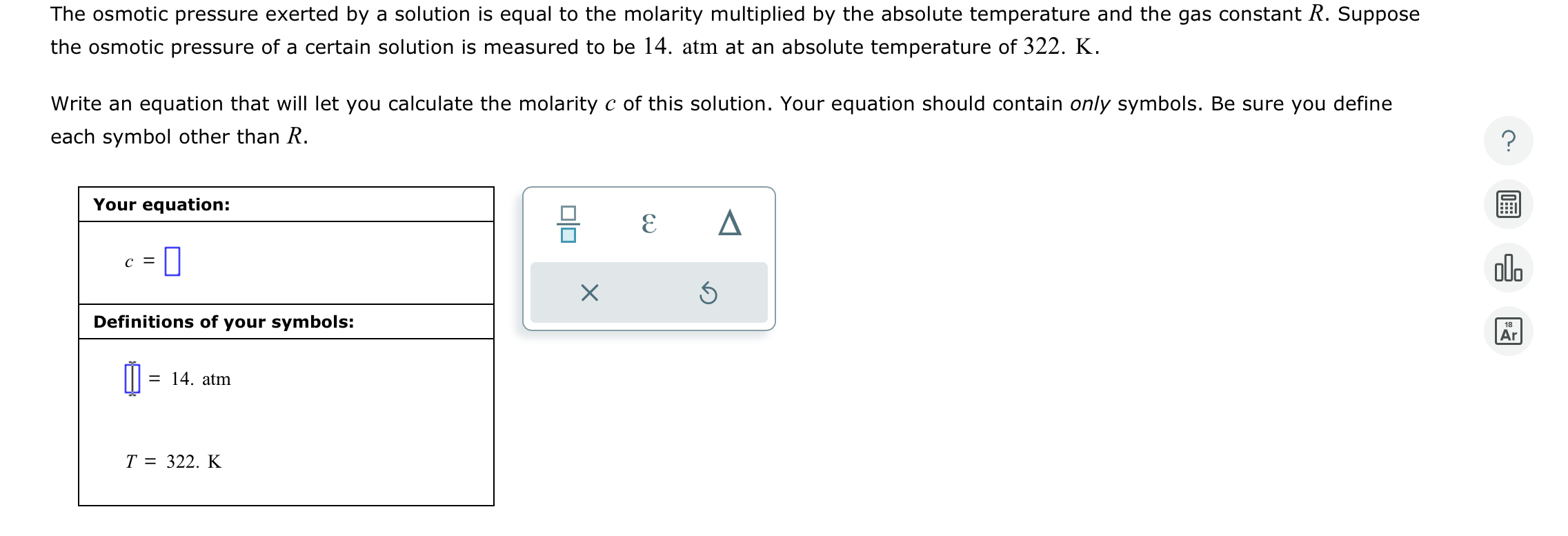
The osmotic pressure exerted by a solution is equal to the molaritymultiplied by the absolute temperature and the gas constant R . Suppose the osmotic
The osmotic pressure exerted by a solution is equal to the molaritymultiplied by the absolute temperature and the gas constant Suppose
the osmotic pressure of a certain solution is measured to be atm at an absolute temperature of K
Write an equation that will let you calculate the molarity of this solution. Your equation should contain only symbols. Be sure you define
each symbol other than
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


