Question
The pair(s) of complexes wherein both exhibit tetrahedral geometry is(are) (Note: py= pyridine Given: Atomic numbers of Fe, Co, Ni and Cu are 26,
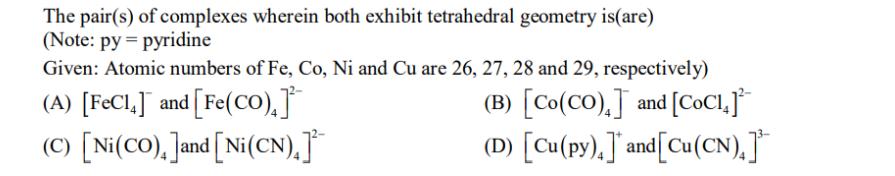
The pair(s) of complexes wherein both exhibit tetrahedral geometry is(are) (Note: py= pyridine Given: Atomic numbers of Fe, Co, Ni and Cu are 26, 27, 28 and 29, respectively) (A) [FeCl,] and [Fe(CO),] (B) [Co(CO),] and [COCI+] (C) [Ni(CO), ]and [Ni(CN).] (D) [Cu(py),]* and[Cu(CN)]
Step by Step Solution
3.46 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Essentials of Materials Science and Engineering
Authors: Donald R. Askeland, Wendelin J. Wright
3rd edition
978-1111576868, 1111576866, 978-1285677620, 1285677625, 978-1111576851
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



