Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The partial molar enthalpies of the two components of a binary mixture at 25C are given by the two following equations: H1=100(1+0.08x22)H2=80(1+0.1x12) where the enthalpy
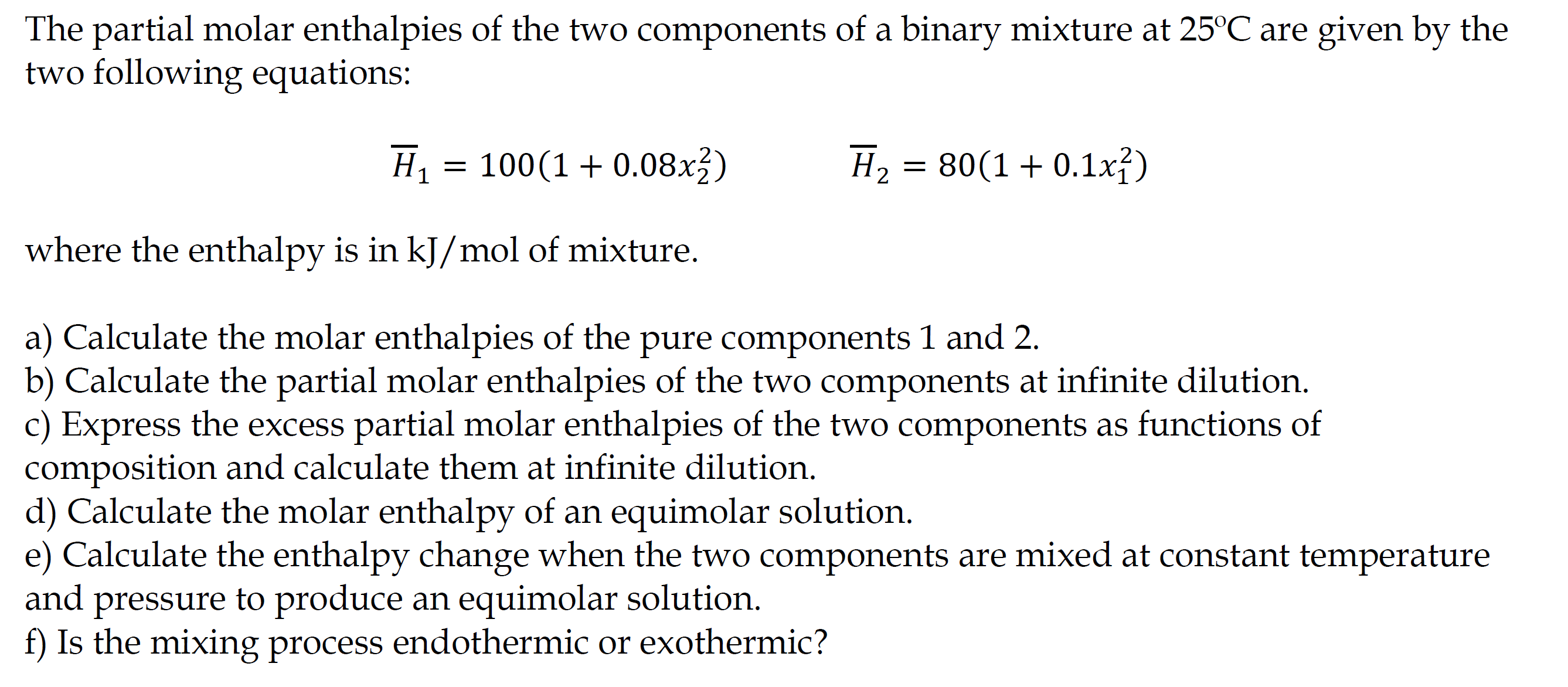 The partial molar enthalpies of the two components of a binary mixture at 25C are given by the two following equations: H1=100(1+0.08x22)H2=80(1+0.1x12) where the enthalpy is in kJ/mol of mixture. a) Calculate the molar enthalpies of the pure components 1 and 2. b) Calculate the partial molar enthalpies of the two components at infinite dilution. c) Express the excess partial molar enthalpies of the two components as functions of composition and calculate them at infinite dilution. d) Calculate the molar enthalpy of an equimolar solution. e) Calculate the enthalpy change when the two components are mixed at constant temperature and pressure to produce an equimolar solution. f) Is the mixing process endothermic or exothermic
The partial molar enthalpies of the two components of a binary mixture at 25C are given by the two following equations: H1=100(1+0.08x22)H2=80(1+0.1x12) where the enthalpy is in kJ/mol of mixture. a) Calculate the molar enthalpies of the pure components 1 and 2. b) Calculate the partial molar enthalpies of the two components at infinite dilution. c) Express the excess partial molar enthalpies of the two components as functions of composition and calculate them at infinite dilution. d) Calculate the molar enthalpy of an equimolar solution. e) Calculate the enthalpy change when the two components are mixed at constant temperature and pressure to produce an equimolar solution. f) Is the mixing process endothermic or exothermic Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


