Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The PH did decrease from 9 to 8 8. Bubble air (carbon dioxide) into the cup for approximately 10 minutes using a drinking straw. You
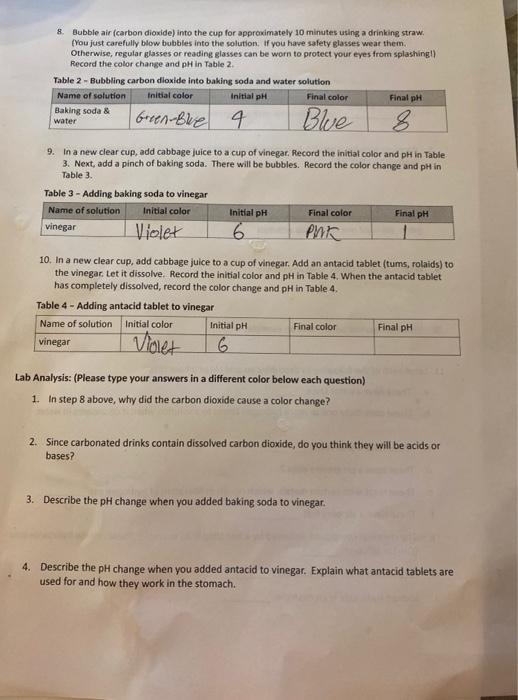

The PH did decrease from 9 to 8
8. Bubble air (carbon dioxide) into the cup for approximately 10 minutes using a drinking straw. You just carefully blow bubbles into the solution. If you have safety glasses wear them. Otherwise, regular glasses or reading glasses can be worn to protect your eyes from splashing!) Record the color change and pH in Table 2 Table 2 - Bubbling carbon dioxide into baking soda and water solution Name of solution Initial color Initial pH Final color Final pH Baking soda & water Green-bre 4 Blue 9. In a new clear cup, add cabbage juice to a cup of vinegar. Record the initial color and pH in Table 3. Next, add a pinch of baking soda. There will be bubbles. Record the color change and pH in Table 3. Table 3 - Adding baking soda to vinegar Name of solution Initial color Initial pH Final color Final pH vinegar Violet 6 Pink 10. In a new clear cup, add cabbage juice to a cup of vinegar. Add an antacid tablet (tums, rolaids) to the vinegar. Let it dissolve. Record the initial color and pH in Table 4. When the antacid tablet has completely dissolved, record the color change and pH in Table 4. Table 4 - Adding antacid tablet to vinegar Name of solution Initial color Initial pH Final color Final pH vinegar 6 Violet Lab Analysis: (Please type your answers in a different color below each question) 1. Instep 8 above, why did the carbon dioxide cause a color change? 2. Since carbonated drinks contain dissolved carbon dioxide, do you think they will be acids or bases? 3. Describe the pH change when you added baking soda to vinegar. 4. Describe the pH change when you added antacid to vinegar. Explain what antacid tablets are used for and how they work in the stomach Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


