Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The population distribution for different energy levels follows the Boltzmann distribution law when the atoms are in the thermal dynamic equilibrium. The number of
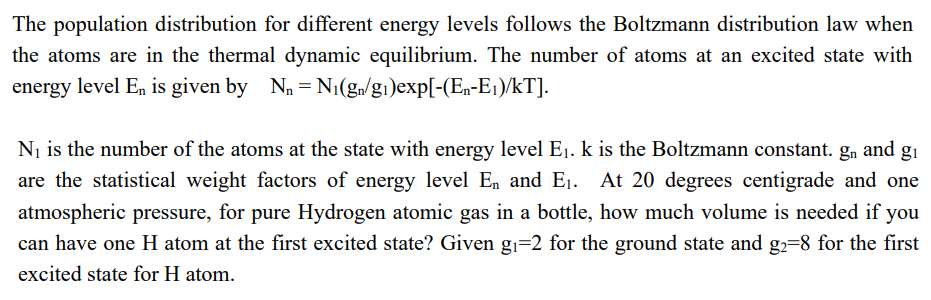
The population distribution for different energy levels follows the Boltzmann distribution law when the atoms are in the thermal dynamic equilibrium. The number of atoms at an excited state with energy level En is given by N = N(gn/g1)exp[-(En-E1)/kT]. N is the number of the atoms at the state with energy level E. k is the Boltzmann constant. gn and g are the statistical weight factors of energy level E and E. At 20 degrees centigrade and one atmospheric pressure, for pure Hydrogen atomic gas in a bottle, how much volume is needed if you can have one H atom at the first excited state? Given g=2 for the ground state and g2=8 for the first excited state for H atom.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To find the volume of the bottle needed to have one hydrogen atom at the first excited state we can ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


