Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The potential energy of two atoms in a diatomic molecule is approximated by U (r) = a/r2-b/6, where r is the spacing between atoms
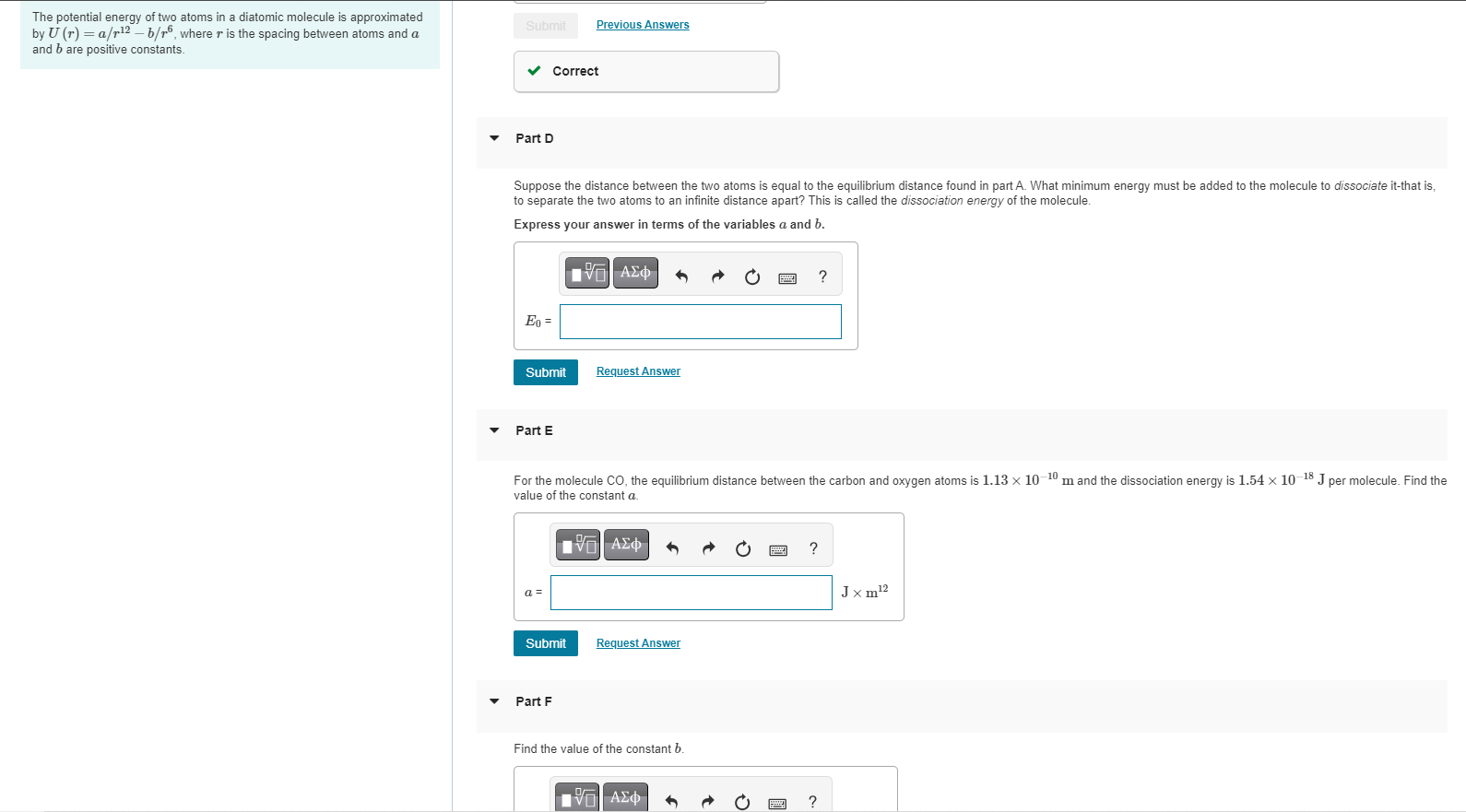
The potential energy of two atoms in a diatomic molecule is approximated by U (r) = a/r2-b/6, where r is the spacing between atoms and a and b are positive constants. Submit Correct Part D Suppose the distance between the two atoms is equal to the equilibrium distance found in part A. What minimum energy must be added to the molecule to dissociate it-that is, to separate the two atoms to an infinite distance apart? This is called the dissociation energy of the molecule. Express your answer in terms of the variables a and b. Eo = Submit Part E Previous Answers a = Submit Part F 17| Request Answer For the molecule CO, the equilibrium distance between the carbon and oxygen atoms is 1.13 x 10-10 m and the dissociation energy is 1.54 x 10-18 J per molecule. Find the value of the constant a. IVE Request Answer Find the value of the constant b. V| P ? www ? ? Jxm2 12
Step by Step Solution
★★★★★
3.45 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
To solve the given problems related to the potential energy function Ur arb we can follow the steps ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


