Answered step by step
Verified Expert Solution
Question
1 Approved Answer
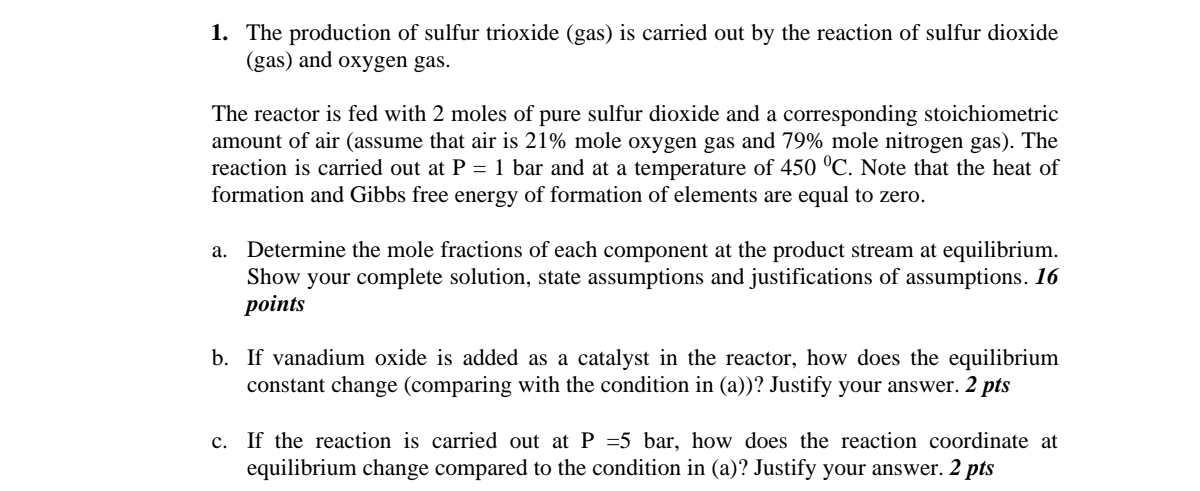
The production of sulfur trioxide ( gas ) is carried out by the reaction of sulfur dioxide ( gas ) and oxygen gas. The reactor
The production of sulfur trioxide gas is carried out by the reaction of sulfur dioxide gas and oxygen gas.
The reactor is fed with moles of pure sulfur dioxide and a corresponding stoichiometric amount of air assume that air is mole oxygen gas and mole nitrogen gas The reaction is carried out at bar and at a temperature of Note that the heat of formation and Gibbs free energy of formation of elements are equal to zero.
a Determine the mole fractions of each component at the product stream at equilibrium. Show your complete solution, state assumptions and justifications of assumptions. points
b If vanadium oxide is added as a catalyst in the reactor, how does the equilibrium constant change comparing with the condition in a Justify your answer. pts
c If the reaction is carried out at bar, how does the reaction coordinate at equilibrium change compared to the condition in a Justify your answer. pts
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


