Answered step by step
Verified Expert Solution
Question
1 Approved Answer
the question has been answered already, I just want to know how did we get 1/[(1000 J/kJ)] in the solution in finding m. I'm sure
the question has been answered already, I just want to know how did we get 1/[(1000 J/kJ)] in the solution in finding m. I'm sure its for the purpose of canceling out units. However, pls explain to me CLEARLY how/why? how did we get kg as unit for mass- and whats the purpose of 1/[(1000 J/kJ)]? 

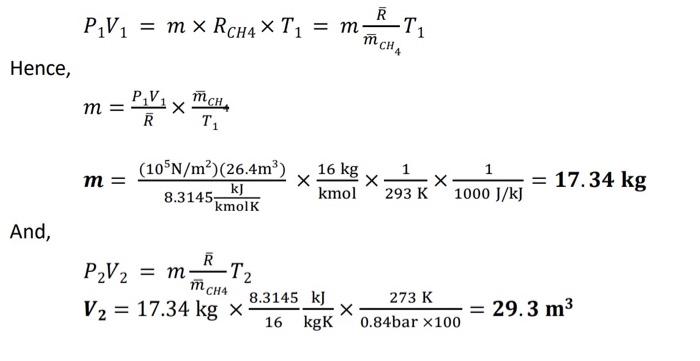
Problem: A balloon is filled with methane gas (CH4) at 20C and 1 bar until the volume is 26.4m3. Calculate the mass of methane under ideal gas conditions. Determine the volume if the balloon rises to a height where the state is 0.84 bar and 0C. Take universal gas constant, R=8.3145kJ/kmolK and molar mass of methane =16kg/kmol. Solution: Given: P1=1barT1=20C=293KV1=26.4m3m=?P2=0.84bar,T2=0C=273K,V2=?R=8.3145kJ/kmolK,mCH$=16kg/kmol P1V1=mRCH4T1=mmC4RT1 Hence, m=RP1V1T1mCHm=8.3145kmolKk(105N/m2)(26.4m3)kmol16kg293K11000J/kJ1=17.34kg And, P2V2=mmCHRT2V2=17.34kg168.3145kgKkJ0.84bar100273K=29.3m3 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


