

the Question is about providing the mechabism if applicable. if it is not applicable then provide the reason.

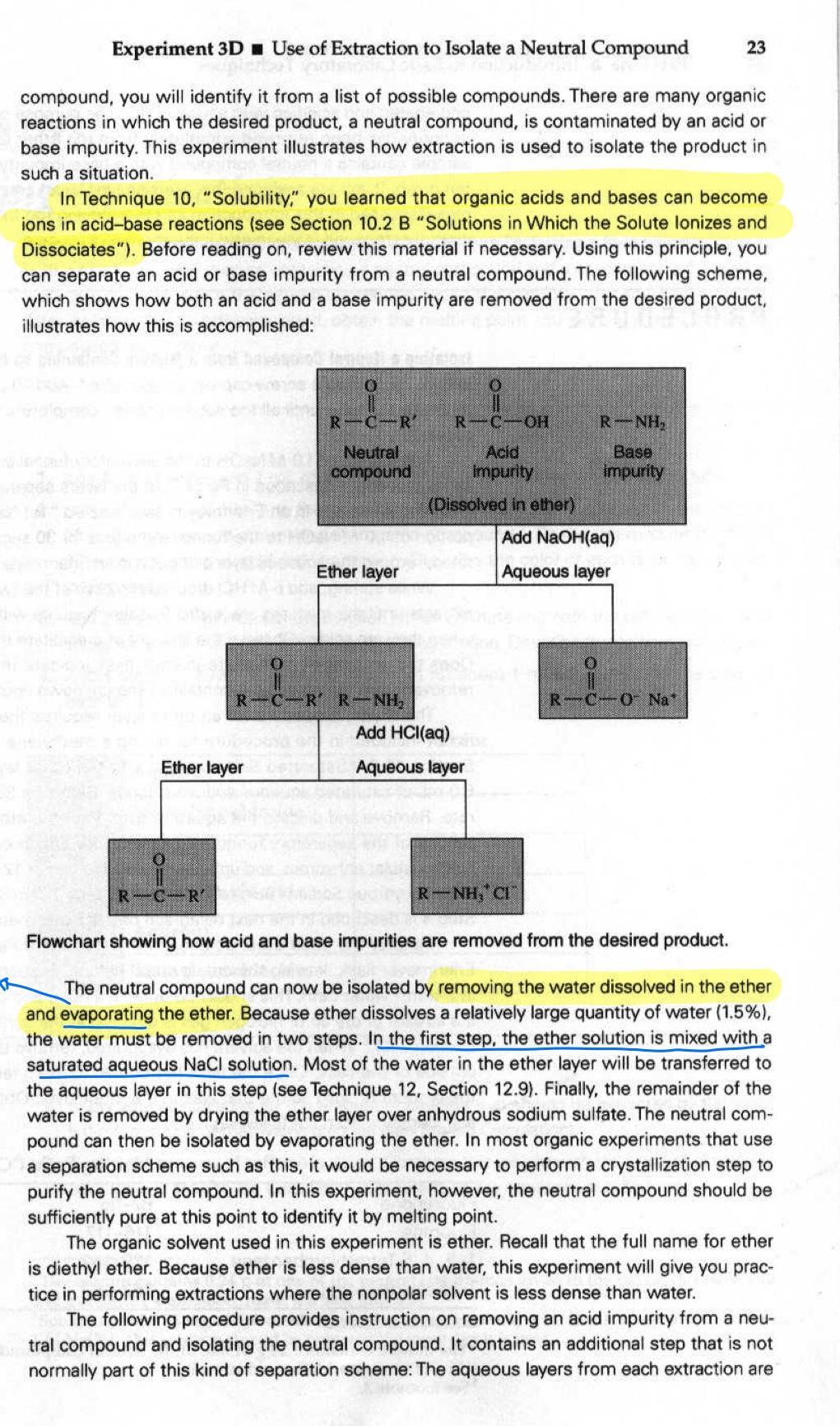
Experiment 3D Use of Extraction to Isolate a Neutral Compound 23 compound, you will identify it from a list of possible compounds. There are many organic reactions in which the desired product, a neutral compound, is contaminated by an acid or base impurity. This experiment illustrates how extraction is used to isolate the product in such a situation. In Technique 10, "Solubility," you learned that organic acids and bases can become ions in acid-base reactions (see Section 10.2 B "Solutions in which the Solute lonizes and Dissociates"). Before reading on, review this material if necessary. Using this principle, you can separate an acid or base impurity from a neutral compound. The following scheme, which shows how both an acid and a base impurity are removed from the desired product, illustrates how this is accomplished: R-CD R-C-OH R-NH, Neutral compound Acid impurity (Dissolved in ether) Base impurity Add NaOH(aq) Ether layer Aqueous layer R-C-0- Na' R-C-RR-NH, Add HCl(aq) Ether layer Aqueous layer R-C-R R - NH, CH Flowchart showing how acid and base impurities are removed from the desired product. The neutral compound can now be isolated by removing the water dissolved in the ether and evaporating the ether. Because ether dissolves a relatively large quantity of water (1.5%), the water must be removed in two steps. In the first step, the ether solution is mixed with a saturated aqueous NaCl solution. Most of the water in the ether layer will be transferred to the aqueous layer in this step (see Technique 12, Section 12.9). Finally, the remainder of the water is removed by drying the ether layer over anhydrous sodium sulfate. The neutral com- pound can then be isolated by evaporating the ether. In most organic experiments that use a separation scheme such as this, it would be necessary to perform a crystallization step to purify the neutral compound. In this experiment, however, the neutral compound should be sufficiently pure at this point to identify it by melting point. The organic solvent used in this experiment is ether. Recall that the full name for ether is diethyl ether. Because ether is less dense than water, this experiment will give you prac- tice in performing extractions where the nonpolar solvent is less dense than water. The following procedure provides instruction on removing an acid impurity from a neu- tral compound and isolating the neutral compound. It contains an additional step that is not normally part of this kind of separation scheme: The aqueous layers from each extraction are segregated and acidified with aqueous HCI. The purpose of this step is to verify that the acid impurity has been removed completely from the ether layer. In the Optional Exercise, the sample contains a neutral compound with a base impurity; however, a detailed procedure is not given. If you are assigned this exercise, you must create a procedure by using the princi- ples discussed in this introduction and by studying the following procedure for isolating the neutral compound from an acid impurity, Isolating a Neutral Compound from a Mixture Containing an Acid Impurity. Add 0.36 g of an un- known mixture to a screw-cap centrifuge tube. Add 10.0 mL of ether to the tube and cap it. Shake the tube until all the solid dissolves completely. Transfer this solution to a 125-ml separatory funnel. Add 5.0 mL of 1.0 M NaOH to the separatory funnel and shake for 30 seconds, using the same procedure described in Part A. Let the layers separate. Remove the bottom (aqueous) layer and place this in an Erlenmeyer flask labeled "1st NaOH extract." Add another 5.0-ml portion of 1.0 M NaOH to the funnel and shake for 30 seconds. When the layers have sepa- rated, remove the aqueous layer and put it in an Erlenmeyer flask labeled "2nd NaOH extract." While stirring, add 6 M HCl dropwise to each of the two test flasks containing the NaOH extracts until the mixtures are acidic. Test the mixtures with litmus or pH paper to determine when they are acidic. Observe the amount of prcipitate that forms. What is the precipitate? Does the amount of precipitate in each flask indicate that all the acid impurity has been removed from the ether layer containing the unknown neutral compound? The drying procedure for an ether layer requires the following additional step, which is not included in the procedure for drying a methylene chloride layer (see Technique 12, Section 12.9,"Saturated Salt Solution"). To the ether layer in the separatory funnel, add 5.0 mL of saturated aqueous sodium chloride. Shake for 30 seconds and let the layers sepa- rate. Remove and discard the aqueous layer. Pour the ether layer (without any water) from the top of the separatory funnel into a clean, dry Erlenmeyer flask. Now dry the ether layer over granular anhydrous sodium sulfate (see Technique 12, Section 12.9. "Drying Procedure with Anhydrous Sodium Sulfate"). Complete steps 1-3 in "A. Macroscale Drying Procedure." Step 4 is described in the next paragraph of this experiment. Transfer the dried ether solution with a clean, dry Pasteur pipet to a dry, preweighed Erlenmeyer flask, leaving the drying agent behind. Evaporate the ether by heating the flask in a warm water bath. This should be done in a hood and can be accomplished more rapidly if a stream of dry air or nitrogen gas is directed at the surface of the liquid (see Technique 7 Section 710). When the solvent has evaporated, remove the flask from the bath and dry the outside of the flask. Once the flask has cooled to room temperature, weigh it to determine the amount of solid solute that was in the ether layer. Obtain the melting point of the solid and identify it from the following table: Melting Point ("C) 82-85 Fluorenone Fluorene 1, 2, 4,5-Tetrachlorobenzene Triphenylmethanol 116-117 139-142 162-164 The minute contains 0.24 g of one of the neutral compounds given in the following table and 0.12 g of benzoic acid, the acid impurity. See footnote 3 3D EXPERIMENT 3D a Use of Extraction to Isolate a Neutral Compound from a Mixture Containing an Acid or Base Impurity In this experiment, you will be given a solid sample containing an unknown neutral com pound and an acid or base impurity. The goal is to remove the acid or base by extrac- tion and isolate the neutral compound. By determining the melting point of the neutral *The three mixtures will likely be (1) water and mi-butyl chloride, (2) water and n-butyl bromide, and (3) n-butyl bromide and saturated aqueous sodium bromide. E. Mechanism (if applicable, must be handwritten, never typed): Write clear stepwise mechanisms for all synthetic transformations, showing important intermediates where appropriate










