Question
The radius of the orbit of an electron in a Hydrogen-like atom is 4.5 ao where ao is the Bohr radius. Its 3h orbital
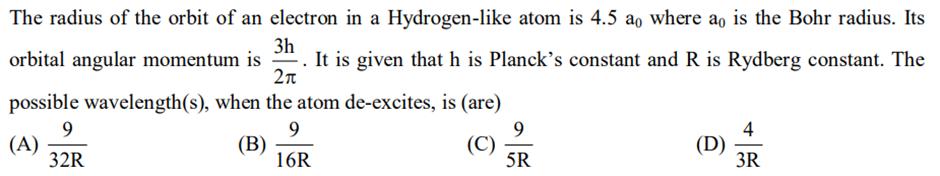
The radius of the orbit of an electron in a Hydrogen-like atom is 4.5 ao where ao is the Bohr radius. Its 3h orbital angular momentum is It is given that h is Planck's constant and R is Rydberg constant. The 2 possible wavelength(s), when the atom de-excites, is (are) 9 (A) (B) (C) 16R 9 32R 9 5R (D) 4 3R
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The detaile...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Ethics for Scientists and Engineers
Authors: Edmund G. Seebauer, Robert L. Barry
1st Edition
9780195698480, 195134885, 195698487, 978-0195134889
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



