Question
An electron in a hydrogen atom is described by the radial wave function given by with energy Hint: R32 (r): 6. = 4 p.
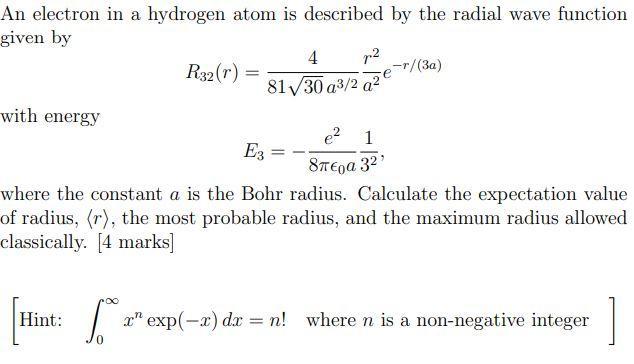
An electron in a hydrogen atom is described by the radial wave function given by with energy Hint: R32 (r): 6. = 4 p. 8130 a3/2 a2 E3 = e 1 8 3 where the constant a is the Bohr radius. Calculate the expectation value of radius, (r), the most probable radius, and the maximum radius allowed classically. [4 marks] -r/(3a) x" exp(-x) dx = n! where n is a non-negative integer
Step by Step Solution
3.43 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
Answer Radial atom espectaction value of 4 fa 16 81x81x3007 R32 2 81x81x30a7 16 818 130 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Managerial economics applications strategy and tactics
Authors: James r. mcguigan, R. Charles Moyer, frederick h. deb harris
12th Edition
9781133008071, 1439079234, 1133008070, 978-1439079232
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



