Answered step by step
Verified Expert Solution
Question
1 Approved Answer
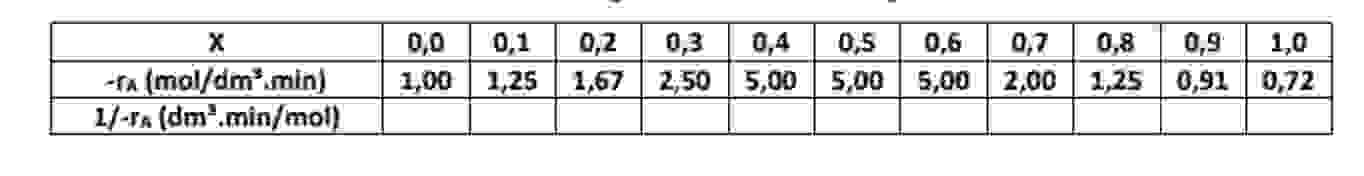
The reaction A + B - > 1 / 2 C + 3 / 2 D It was conducted isothermally, and the following data were
The reaction
A B C D
It was conducted isothermally, and the following data were recorded:
a How large would a CSTR be to achieve conversion?
b Repeat the calculation for a PFR reactor.
c Considering that a PFR reactor was added in series after the CSTR reactor in letter a what is the
volume needed to achieve conversion?
d What is the maximum conversion to be achieved if a CSTR added in series with the first PFR
has a volume of dm The inlet molar flow rate of A was molmintableXramoldmminFAAdmminmol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


