Answered step by step
Verified Expert Solution
Question
1 Approved Answer
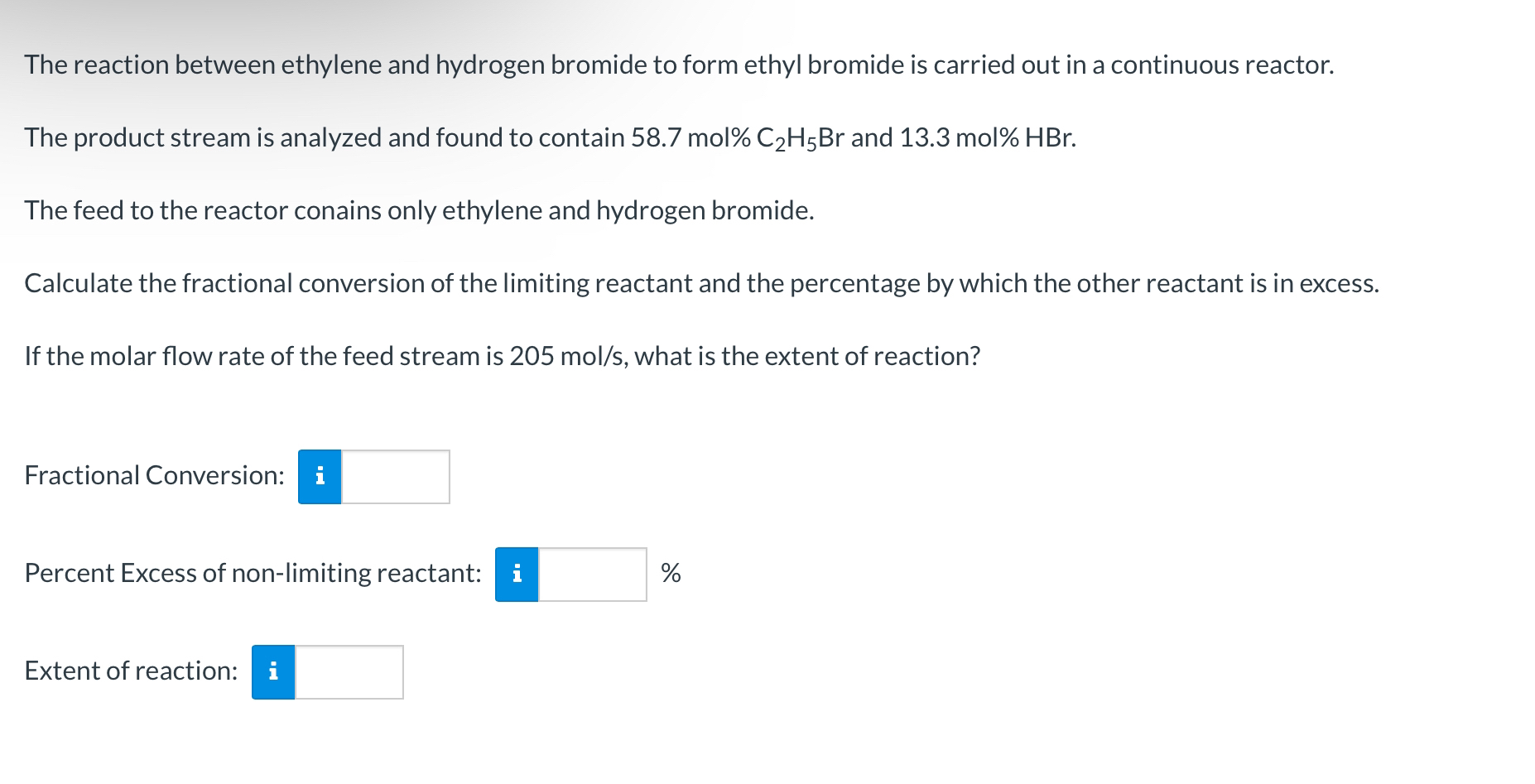
The reaction between ethylene and hydrogen bromide to form ethyl bromide is carried out in a continuous reactor. The product stream is analyzed and found
The reaction between ethylene and hydrogen bromide to form ethyl bromide is carried out in a continuous reactor.
The product stream is analyzed and found to contain mol and mol
The feed to the reactor conains only ethylene and hydrogen bromide.
Calculate the fractional conversion of the limiting reactant and the percentage by which the other reactant is in excess.
If the molar flow rate of the feed stream is what is the extent of reaction?
Fractional Conversion:
Percent Excess of nonlimiting reactant:
Extent of reaction:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


