Question
The reaction: C6H5CH3+H2 C6H6+CH4 (T+H2 B+M) was studied on Al2O3-SiO2 catalyst. During the study the following observations were made: 1) The reaction is essentially irreversible.
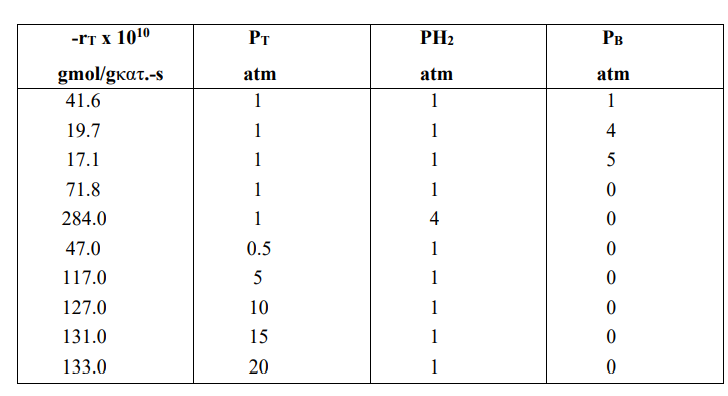
The reaction: C6H5CH3+H2 C6H6+CH4 (T+H2 B+M) was studied on Al2O3-SiO2 catalyst. During the study the following observations were made: 1) The reaction is essentially irreversible. 2) The partial pressure of methane does not affect the rate of the reaction. 3) The rate decreases with increasing partial pressure of benzene. 4) At low concentrations of toluene, the rate increases with increasing partial pressure of toluene and remains almost constant at high concentrations. 5) The rate increases linearly with increasing hydrogen concentration. a) Based on the above observations, write the most likely reaction mechanism. b) If the regulating step is the surface reaction, develop a rate equation based on the mechanism of (a) in terms of partial pressures. c) Calculate the constants that appear in the rate equation based on the following experimental data.
 d) A CSTR reactor has a volume of 0.2L and contains a solution of Pd nanoparticles of concentration 4 mol/L. The Pd nanoparticles are the catalyst for a reaction and the turnover frequency (TOF) is 7 moles of product produced per second per mole of Pd. i) What is the reaction rate of this reactor in moles of product per liter per second? ii) 4 g of catalyst are used in one reaction, the catalyst is 2 wt%. Au on a TiO2 support (the active sites are the gold atoms), the dispersion of exposed Au atoms (Dispersion, D) is 75% and the amount of product produced per second is 0.3 mol/s. Find the TOF.
d) A CSTR reactor has a volume of 0.2L and contains a solution of Pd nanoparticles of concentration 4 mol/L. The Pd nanoparticles are the catalyst for a reaction and the turnover frequency (TOF) is 7 moles of product produced per second per mole of Pd. i) What is the reaction rate of this reactor in moles of product per liter per second? ii) 4 g of catalyst are used in one reaction, the catalyst is 2 wt%. Au on a TiO2 support (the active sites are the gold atoms), the dispersion of exposed Au atoms (Dispersion, D) is 75% and the amount of product produced per second is 0.3 mol/s. Find the TOF.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


