Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The reaction given above is the elementary reaction and k = 250d * m ^ 6 d * m ^ 6 / m * o
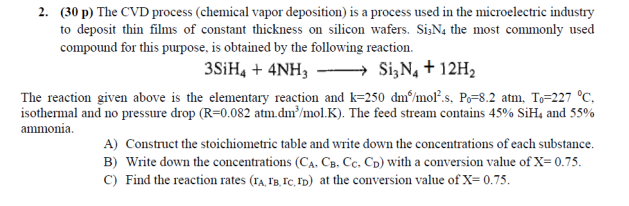
The reaction given above is the elementary reaction and k = 250d * m ^ 6 d * m ^ 6 / m * o * l ^ 2 , P_{0} = 8.2atm T_{0} = 227 deg * C isothermal and no pressure drop (R=0.082 atm. d * m ^ 3 / m * ol .K). The feed stream contains 45\% Si*H_{4} and 55% ammonia.
A) Construct the stoichiometric table and write down the concentrations of each substance.
B) Write down the concentrations ( C_{A} .C B .C C .C D ) with a conversion value of X = 0.75 C) Find the reaction rates (r_{A}, r_{B}, r_{C}, r_{D}) at the conversion value of X = 0.75
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


