Question
The reaction of iron (II) sulfide in the presence of oxygen produces Fe203 and S02 according to the following reaction: Fes + 02 Fe203
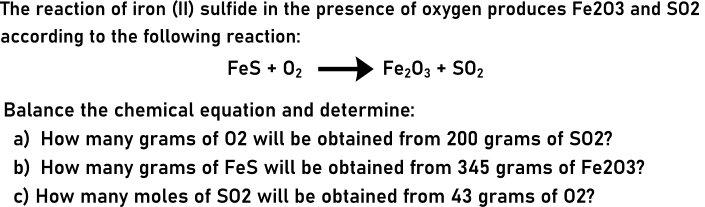
The reaction of iron (II) sulfide in the presence of oxygen produces Fe203 and S02 according to the following reaction: Fes + 02 Fe203 + S02 Balance the chemical equation and determine: a) How many grams of 02 will be obtained from 200 grams of S02? b) How many grams of FeS will be obtained from 345 grams of Fe203? c) How many moles of S02 will be obtained from 43 grams of 02?
Step by Step Solution
3.44 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
Consider the balonced reoction 4fes 702 2fe203 h502 Mole of So ot of soz 200 gm 3D 3 125 mo...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Joseph M. Hornback
2nd Edition
9781133384847, 9780199270293, 534389511, 1133384846, 978-0534389512
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



