The Redlich-Kwong equation of state is given by RT a v(v +b)/T where R = the universal gas constant [= 0.518 kJ/(kg K)], T
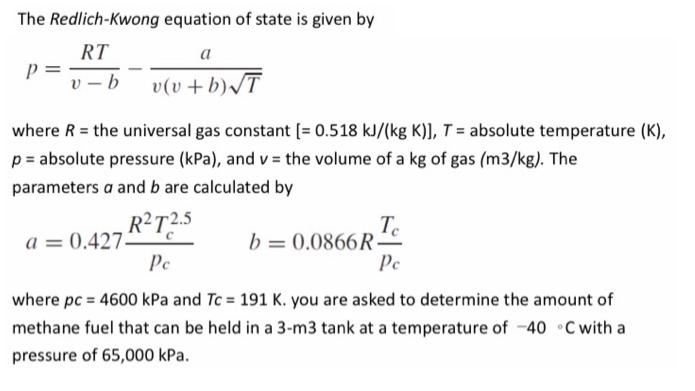
The Redlich-Kwong equation of state is given by RT a v(v +b)/T where R = the universal gas constant [= 0.518 kJ/(kg K)], T = absolute temperature (K), p = absolute pressure (kPa), and v = the volume of a kg of gas (m3/kg). The parameters a and b are calculated by RT2.5 a = 0.427. Pe Te b = 0.0866R- Pe where pc 4600 kPa and Tc = 191 K. you are asked to determine the amount of methane fuel that can be held in a 3-m3 tank at a temperature of -40 C with a pressure of 65,000 kPa.
Step by Step Solution
3.48 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
Answer NB link httpsw...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


