The Redlich-Kwong equation of state is given by RT P= v-b v(v+b)T where R= gas constant for methane (0.518 kJ/(kg K) T= absolute temperature,
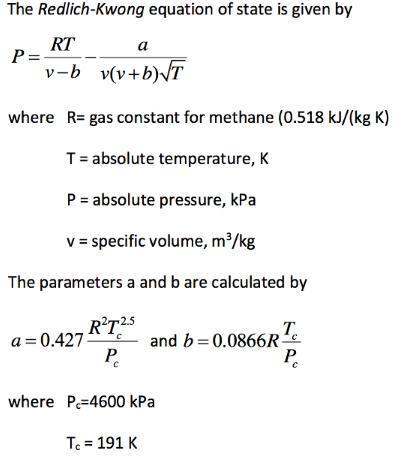

The Redlich-Kwong equation of state is given by RT P= v-b v(v+b)T where R= gas constant for methane (0.518 kJ/(kg K) T= absolute temperature, K P = absolute pressure, kPa v = specific volume, m/kg The parameters a and b are calculated by R'T25 a = 0.427 T. and b=0.0866R- P. P. where P=4600 kPa Te = 191 K As a chemical engineer you are asked to determine the amount of methane fuel that can be held in a 3 m tank at a temperature of -40 C with a pressure of 65,000 kPa. Determine the value of v, and then determine the mass of methane contained in the tank. (Note that you need to change T to an absolute temperature) a) Solve this completely using the symbolic algebra capability in MATLAB. b) Solve using a numerical method of your choice. The root is between 0.0025 and 0.004 m^3/kg. Assign a convergence factor in your calculations, e, such that the answer will have at least 3 significant figures.
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


