The Redlich/Kwong Equation of State is RT P= vbTV (V+b) where I is the temperature, V is the molar volume, R, is the universal
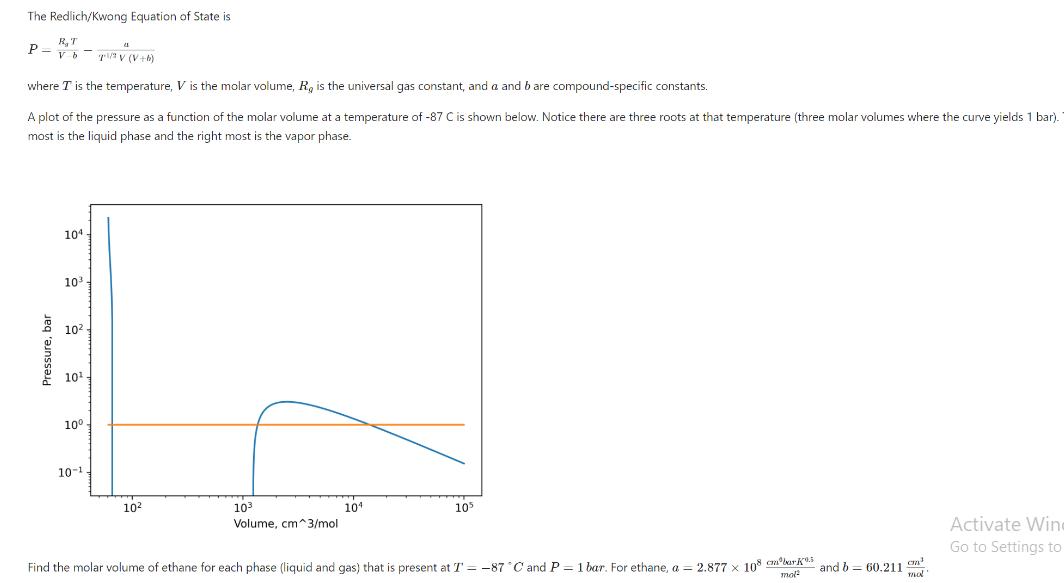
The Redlich/Kwong Equation of State is RT P= vbTV (V+b) where I is the temperature, V is the molar volume, R, is the universal gas constant, and a and b are compound-specific constants. A plot of the pressure as a function of the molar volume at a temperature of -87 C is shown below. Notice there are three roots at that temperature (three molar volumes where the curve yields 1 bar). most is the liquid phase and the right most is the vapor phase. Pressure, bar 104 10 10 10. 10 10-1. 41 10 10 Volume, cm^3/mol 104 105 Find the molar volume of ethane for each phase (liquid and gas) that is present at T = -87 C and P = 1 bar. For ethane, a = 2.877 x 108 cmbark.5 and b 60.211 mol mal Activate Win Go to Settings to
Step by Step Solution
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


