Question
The relative acidity of compounds is influenced by factors including periodic trends, resonance, inductions, and orbital. For each case below there are blue - highlighted
The relative acidity of compounds is influenced by factors including periodic trends, resonance, inductions, and orbital. For each case below there are bluehighlighted protons. Indicate which is the most acidic and briefly explain the factor that is responsible. If resonance is the determining factor, draw relevant resonance structures to illustrate your claim.
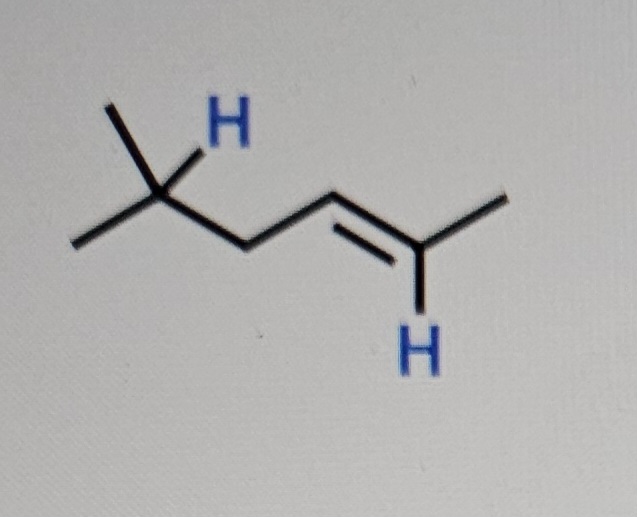
H H
Step by Step Solution
There are 3 Steps involved in it
Step: 1
In the molecule shown in the image there are two highlighted hydrogen atoms positioned on adjacent carbon atoms To determine which hydrogen is more ac...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry A Molecular Approach
Authors: Nivaldo Tro
5th Edition
0134874374, 978-0134874371
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



