Consider the reaction: If a mixture of solid nickel(II) oxide and 0.20 M carbon monoxide comes to
Question:
Consider the reaction: 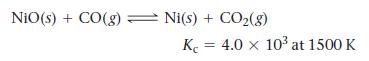
If a mixture of solid nickel(II) oxide and 0.20 M carbon monoxide comes to equilibrium at 1500 K, what is the equilibrium concentration of CO2?
Transcribed Image Text:
NiO(s) + CO(g) Ni(s) + CO₂(g) K 4.0 x 10³ at 1500 K =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A container has a double wall where the wall cavity is filled with carbon dioxide at room temperature and pressure. When the container is filled with a cryogenic liquid at 100 K the carbon dioxide...
-
A container has a double wall where the wall cavity is filled with carbon dioxide at room temperature and pressure. When the container is filled with a cryogenic liquid at 100 K the carbon dioxide...
-
The formation of tetrafluoroethylene from its elements is highly exothermic: (a) If a mixture of F2, graphite, and C2F4 is at equilibrium in a closed container, will the reaction go to the right or...
-
Find the magnitude and direction of the electric field strength at the point P due to the point charges at A and B as shown in the Figure 1. (k = 9 x 10 Nm/C) +2 C 10 cm A 10 cm Figure 1 B -8 C (5...
-
What is an operational productivity measure? A financial measure?
-
The figure is a stereogram for an orthorhombic lattice \((\alpha=\beta=\gamma=\) \(90^{\circ}, a eq b eq c\) ). Including rotations and flips, what symmetry operations does the stereogram show?
-
Financial information from the records of Blanchard Masonry follows. The company began operations in 1994. Assume that the year-end 1994 balances are the average balances during 1994. 1997 1996 1995...
-
The Stopdecay Company sells an electric toothbrush for $25. Its sales have averaged 8,000 units per month over the past year. Recently, its closest competitor, Decayfighter, reduced the price of its...
-
Darcy Roofing is faced with a decision. The company relies very heavily on the use of its 60-foot extension lift for work on large homes and commercial properties. Last year, Darcy Roofing spent...
-
Howard McGraw of Windsor opened First City Surveying Service. As his accountant, analyze the transactions listed and present to Howard the following information, in proper form. 1. The analysis of...
-
For the reaction shown here, K c = 255 at 1000 K. CO(g) + Cl 2 (g) COCl 2 (g) If a reaction mixture initially contains a CO concentration of 0.1500 M and a Cl 2 concentration of 0.175 M at 1000 K,...
-
For the reaction shown here, K c = 0.513 at 500 K. N 2 O 4 ( g) 2 NO 2 (g) If a reaction vessel initially contains an N 2 O 4 concentration of 0.0500 M at 500 K, what are the equilibrium...
-
Evaluate each expression for a = -3, b = 64, and c = 6. a3 + 2c -Vb
-
Smith & Chief Ltd. of Sydney, Australia, is a merchandising firm that is the sole distributor of a product that is increasing in popularity among Australian consumers. The company's income statements...
-
C. In lab, you measure the x & y components of a possible incompressible flow field as u = 2cxy; and where cand a are constants. v = c(a + x - y) 5. (04 pts) Short answer, what is necessary for the...
-
Year 5% 6% 4 3.546 3.465 5 7% 3.387 3.312 4.329 4.212 4.100 8% 3.993 5.076 4.917 4.767 4.623 Present Value of an Annuity of $1 at Compound Interest 9% 10% 11% 12% 13% 14% 15% 3.240 3.170 3.102 3.037...
-
2. Determine the overturning stability of the cantilever retaining wall shown. The equivalent fluid density is 5.5 kN/m, soil density is 18 kN/m, and the concrete weighs 23.5 kN/m. (5 pts) 2 m 2 m 2...
-
A. For a certain two-dimensional, incompressible flow field the velocity component in the y direction is given by v = 3xy + xy 1. (05 pts) Short answer, what is the condition for this flow field to...
-
We observe that current price of a zero-coupon bond with one year maturity is $0.97 (paying $1 at t = 1). We also observe the current implied forward rate is 2.5%. Under the forward measure, price a...
-
Accounting policies and practices that are most important to the portrayal of the companys financial condition and results, and require managements most difficult, subjective, or complex judgments...
-
Two blocks of mass m 1 = 2.5 kg and m 2 = 3.5 kg rest on a double inclined plane with equal angles (Fig. P4.87). The blocks are connected by a string that passes over a pulley, and the blocks are in...
-
A person riding a bicycle on level ground at a speed of 10 m/s throws a baseball forward at a speed of 15 m/s relative to the bicycle at an angle of 35 relative to the horizontal (x) direction. (a)...
-
A car travels along a level road with speed v = 25 m/s (about 50 mi/h). The coefficient of kinetic friction between the tires and the pavement is K = 0.55. (a) If the driver applies the brakes and...
-
Perfect Paints Ltd manufactures decorative paint at its factory in Wadeville, Johannesburg. Two basic product ranges are manufactured, namely the ProTouch and BestGuard ranges. Paint is sold in 20...
-
Use the following information for the next 2 questions (\#23 \& 24): Question 23 What is the Net Income? 10,000,00050,000,00060,000,00090,000,000 none of the above
-
What would be indicated if a companys return on assets was steady but its return on equity increased rapidly?

Study smarter with the SolutionInn App


