Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The same amount of work W =1.50kJ is being done on one mole of a monatomic gas initially at (V=25.0L,T=293K) through two different reversible

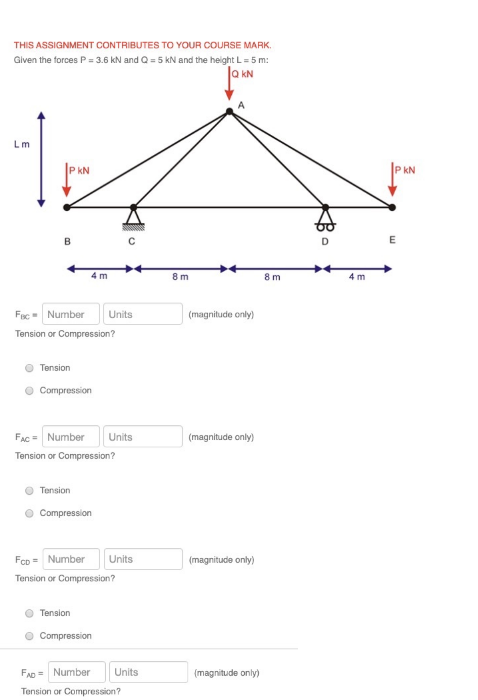
The same amount of work W =1.50kJ is being done on one mole of a monatomic gas initially at (V=25.0L,T=293K) through two different reversible compression processes: isothermal compression and adiabatic compression. a) Calculate the final temperature of the gas for the two compression processes. b) Calculate the final volume of the gas for the two compression processes? c) Calculate the entropy change resulted from the two compression processes? THIS ASSIGNMENT CONTRIBUTES TO YOUR COURSE MARK. Given the forces P = 3.6 kN and Q = 5 kN and the height L = 5 m: Q KN Lm B KN 4 m Fac Number Units Tension or Compression? Tension Compression FAC Number 8 m A (magnitude only) Units (magnitude only) Tension or Compression? Tension Compression Fcp Number Units (magnitude only) Tension or Compression? Tension Compression FAD=Number Units (magnitude only) Tension or Compression? 8 m D E m KN
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


