Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The second order irreversible A B is carried out in a packed bed reactor. Pure A enters at a volumetric flowrate of 0 . 1
The second order irreversible is carried out in a packed bed reactor. Pure A enters at a volumetric flowrate of and the reaction takes place at and kPa, with the rate constant per unit area, is given as s The pure is to be passed over a packed bed of cylindrical particles in diameter and length, whereas the bulk density of the catalyst is with the void fraction The dynamic viscosity is given as Gas diffusivity Effective diffusivity Calculate the mass transfer coefficient, by using ThoenesKramers correlation if the freestream velocity, is Given the equation: Volume average particle diameter, ; 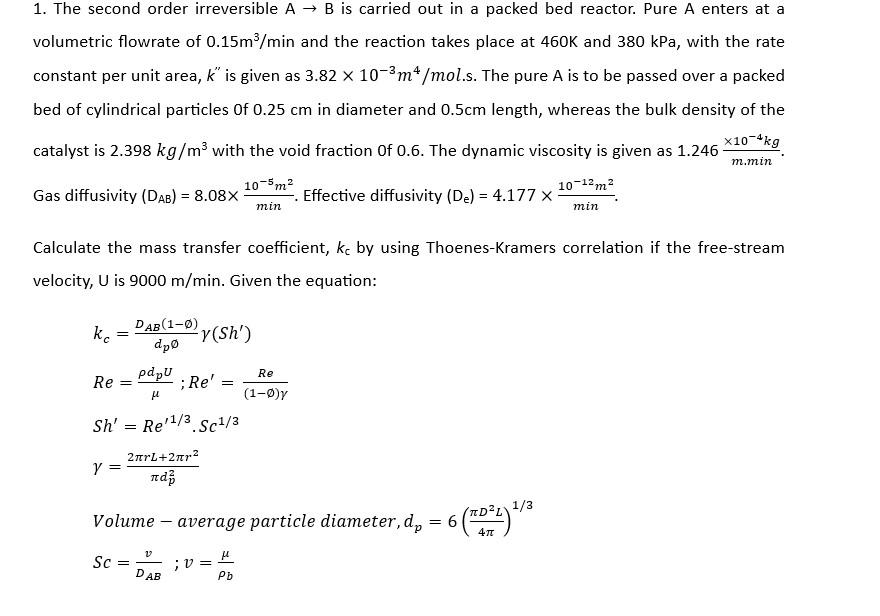
The second order irreversible is carried out in a packed bed reactor. Pure A enters at a
volumetric flowrate of and the reaction takes place at and kPa, with the rate
constant per unit area, is given as s The pure is to be passed over a packed
bed of cylindrical particles in diameter and length, whereas the bulk density of the
catalyst is with the void fraction The dynamic viscosity is given as
Gas diffusivity Effective diffusivity
Calculate the mass transfer coefficient, by using ThoenesKramers correlation if the freestream
velocity, is Given the equation:
Volume average particle diameter,
;
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access with AI-Powered Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


