Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The series of heat exchangers shown in Figure P5.33 is designed to raise a liquid's temperature so a desired chemical reaction can take place
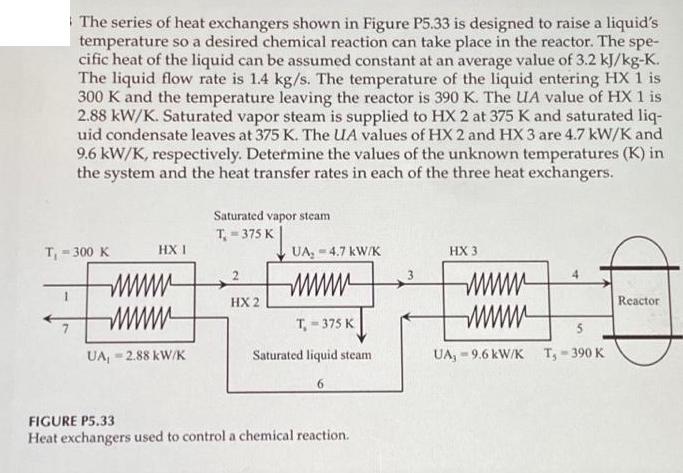
The series of heat exchangers shown in Figure P5.33 is designed to raise a liquid's temperature so a desired chemical reaction can take place in the reactor. The spe- cific heat of the liquid can be assumed constant at an average value of 3.2 kJ/kg-K. The liquid flow rate is 1.4 kg/s. The temperature of the liquid entering HX 1 is 300 K and the temperature leaving the reactor is 390 K. The UA value of HX 1 is 2.88 kW/K. Saturated vapor steam is supplied to HX 2 at 375 K and saturated liq- uid condensate leaves at 375 K. The UA values of HX 2 and HX 3 are 4.7 kW/K and 9.6 kW/K, respectively. Determine the values of the unknown temperatures (K) in the system and the heat transfer rates in each of the three heat exchangers. T-300 K 1 HX I wwwwww UA, -2.88 kW/K Saturated vapor steam T=375 K 2 HX 2 UA, 4.7 kW/K wwwwww T-375 K Saturated liquid steam 6 FIGURE P5.33 Heat exchangers used to control a chemical reaction. HX 3 5 - UA, 9.6 kW/K T, 390 K Reactor
Step by Step Solution
★★★★★
3.30 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
Okay here are the stepbystep calculations 1 Given Liquid flow rate 14 kgs Specific heat ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


