Question
The solubility of a crystalline substance, Compound x , in grams per 100g water is shown in the below. Which of the statements below is
The solubility of a crystalline substance, Compound
x, in grams per
100gwater is shown in the below. Which of the statements below is FALSE?\ Solubility of Compound
x\ a. The conditions corresponding to Point
Drepresent a saturated solution.\ b. The conditions corresponding to Point
Crepresent a supersaturated solution.\ c. When a solution starting at Point
Ais cooled down to
20\\\\deg C,5gof Compound
xper
100gwater will precipitate if crystallization can occur.\ d The solution represented by Point
Ahas a higher concentration than the solution represented by Point
B.\ e. As temperature increases, the solubility of Compound
xalso increases.
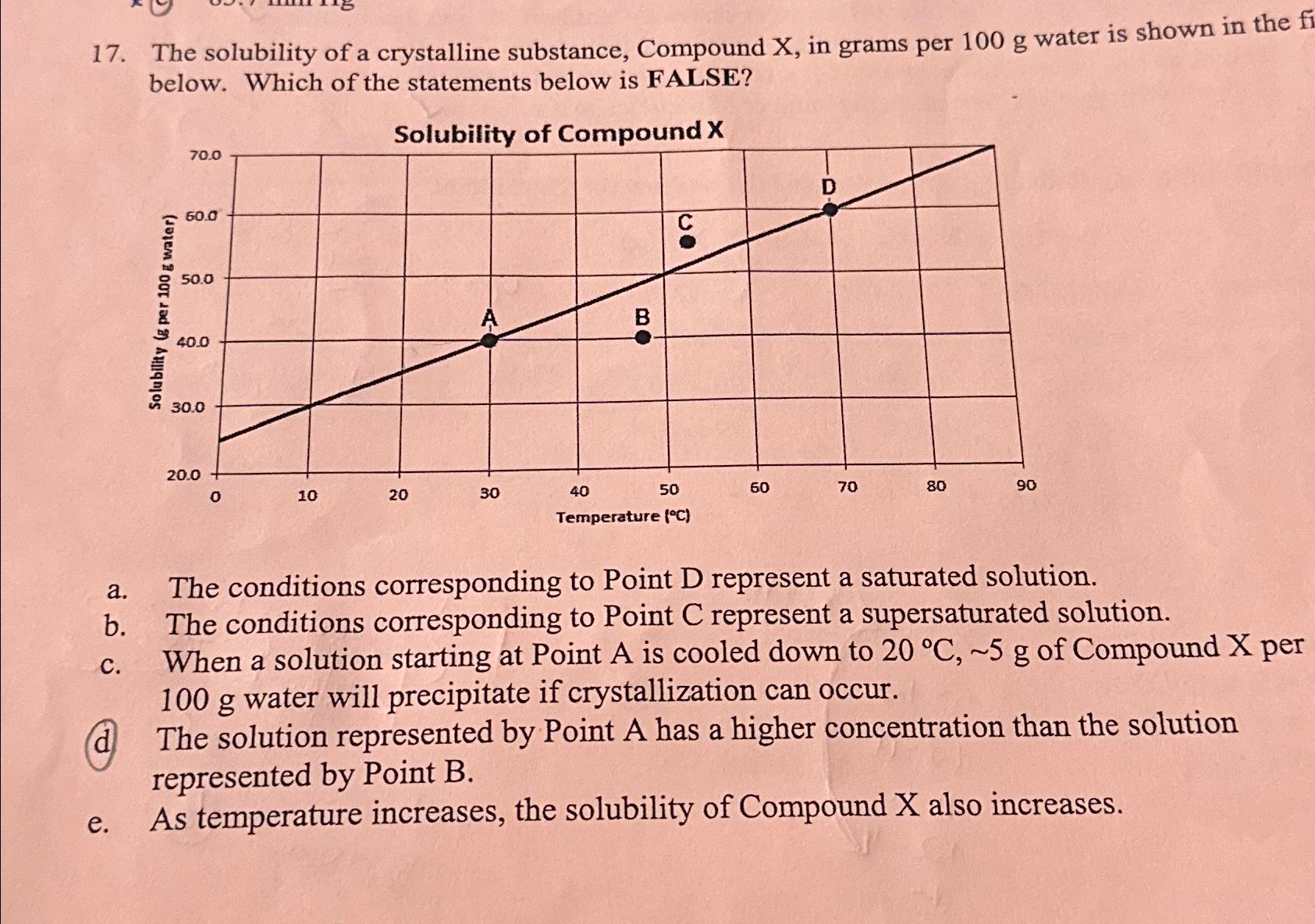
17. The solubility of a crystalline substance, Compound X, in grams per 100 g water is shown in the fi below. Which of the statements below is FALSE? Solubility (g per 100 g water) 70.0 60.0 50.0 40.0 30.0 20.0 Solubility of Compound X D 10 20 30 40 00 BO 50 60 70 80 90 a. b. C. d e. Temperature (C) The conditions corresponding to Point D represent a saturated solution. The conditions corresponding to Point C represent a supersaturated solution. When a solution starting at Point A is cooled down to 20 C, ~5 g of Compound X per 100 g water will precipitate if crystallization can occur. The solution represented by Point A has a higher concentration than the solution represented by Point B. As temperature increases, the solubility of Compound X also increases.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


