Question
The standard potentials of the two half-cells A++, A** | Pt and B**, B* | Pt are Ej and E, respectively. If E2 is
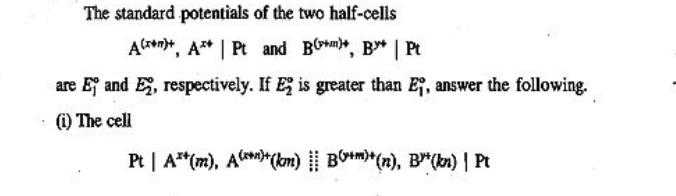

The standard potentials of the two half-cells A++, A** | Pt and B**, B* | Pt are Ej and E, respectively. If E2 is greater than E, answer the following. (i) The cell Pt | A**(m), A()+(km) B+)+(n), B(kn) | Pt was constructed, where k has some constant value. The cell was operated reversibly up to its exhaustion point, i.e. to a point where no more reaction in the cell takes place and hence no more current can be withdrawn from the cell. Show that (a) the half-cell potentials at the exhaustion point are given by the expression nE + ME (n + m) (b) The value of k at the exhaustion point at 298 K is given by the relation nm(E-E) (0.059 13 V)(m + n) logk (ii) Which of the following two cells will give a spontaneous cell reaction when k
Step by Step Solution
3.29 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physical Chemistry
Authors: Peter Atkins
7th Edition
978-0716735397, 716735393, 978-0716743880
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



