Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The standard state Gibbs free energies of formation of C(graphite) and C(diamond) at T=298K are fG0[C(graphite)]=0kJmol1fG0[C(diamond)]=2.9kJmol1 The standard state means that the pressure should be
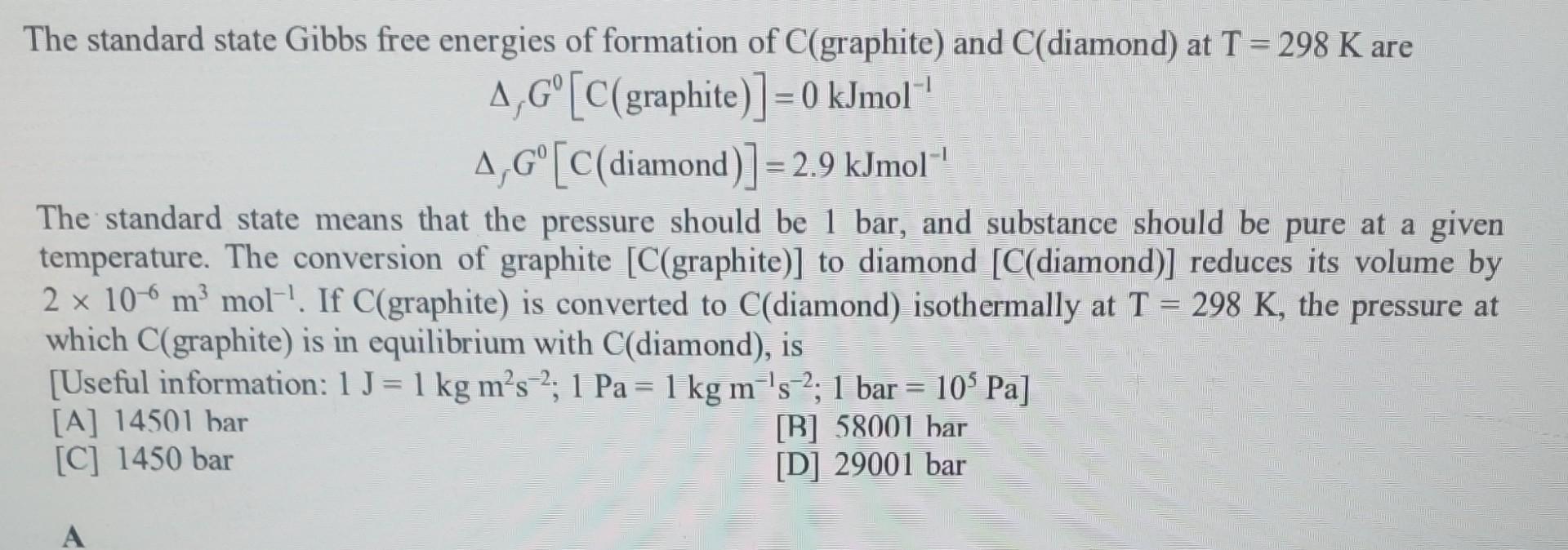
The standard state Gibbs free energies of formation of C(graphite) and C(diamond) at T=298K are fG0[C(graphite)]=0kJmol1fG0[C(diamond)]=2.9kJmol1 The standard state means that the pressure should be 1 bar, and substance should be pure at a given temperature. The conversion of graphite [C(graphite)] to diamond [C(diamond)] reduces its volume by 2106m3mol1. If C (graphite) is converted to C (diamond) isothermally at T=298K, the pressure at which C (graphite) is in equilibrium with C (diamond), is [Useful information: 1J=1kgm2s2;1Pa=1kgm1s2;1bar=105Pa ] [A] 14501 har [B] 58001 har [C] 1450bar [D] 29001bar
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


