Question: The steam table is from Appendix A.III (pages 977-986) book Sandler, Stanley I. - Chemical, biochemical and engineering thermodynamics-Wiley (2017). I couldn't find a good
The steam table is from Appendix A.III (pages 977-986) book "Sandler, Stanley I. - Chemical, biochemical and engineering thermodynamics-Wiley (2017)". I couldn't find a good way to upload it.
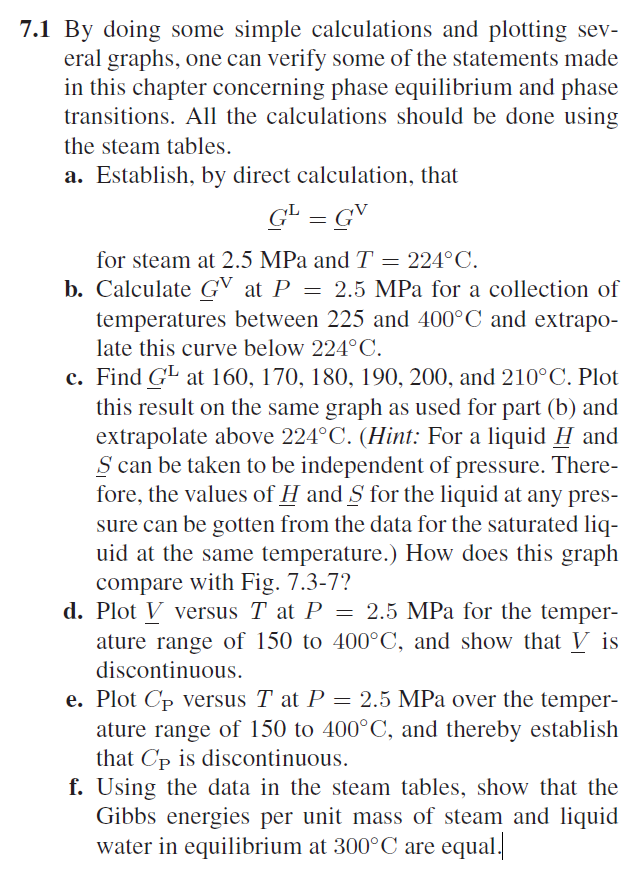
7.1 By doing some simple calculations and plotting several graphs, one can verify some of the statements made in this chapter concerning phase equilibrium and phase transitions. All the calculations should be done using the steam tables. a. Establish, by direct calculation, that GL=GV for steam at 2.5MPa and T=224C. b. Calculate GV at P=2.5MPa for a collection of temperatures between 225 and 400C and extrapolate this curve below 224C. c. Find GL at 160,170,180,190,200, and 210C. Plot this result on the same graph as used for part (b) and extrapolate above 224C. (Hint: For a liquid H and S can be taken to be independent of pressure. Therefore, the values of H and S for the liquid at any pressure can be gotten from the data for the saturated liquid at the same temperature.) How does this graph compare with Fig. 7.3-7? d. Plot V versus T at P=2.5MPa for the temperature range of 150 to 400C, and show that V is discontinuous. e. Plot CP versus T at P=2.5MPa over the temperature range of 150 to 400C, and thereby establish that CP is discontinuous. f. Using the data in the steam tables, show that the Gibbs energies per unit mass of steam and liquid water in equilibrium at 300C are equal
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


