Answered step by step
Verified Expert Solution
Question
1 Approved Answer
the student id is 3,8,9 2. An ammonia stream of 100 mol/min at 25 C and1 atm is fed to a reactor. Meanwhile, an air
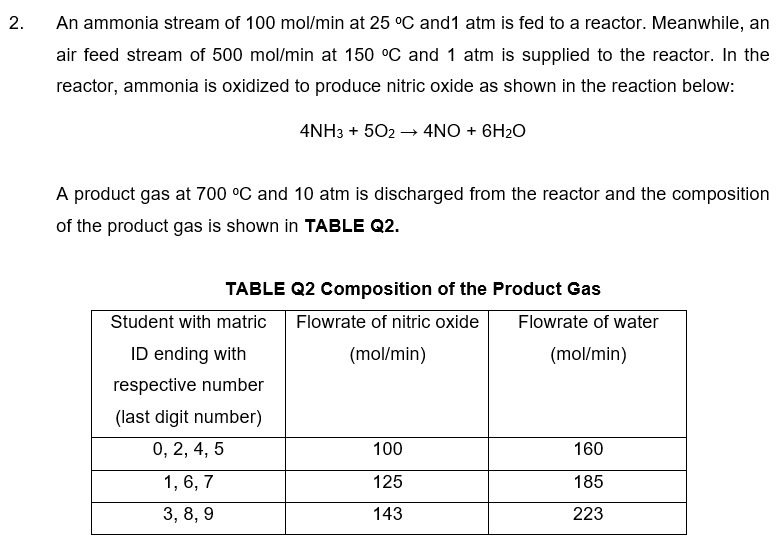

the student id is 3,8,9
2. An ammonia stream of 100 mol/min at 25 C and1 atm is fed to a reactor. Meanwhile, an air feed stream of 500 mol/min at 150 C and 1 atm is supplied to the reactor. In the reactor, ammonia is oxidized to produce nitric oxide as shown in the reaction below: 4NH3 + 502 4NO + 6H20 A product gas at 700 C and 10 atm is discharged from the reactor and the composition of the product gas is shown in TABLE Q2. TABLE Q2 Composition of the Product Gas Student with matric Flowrate of nitric oxide Flowrate of water ID ending with (mol/min) (mol/min) respective number (last digit number) 0, 2, 4, 5 100 160 125 185 1, 6, 7 3, 8, 9 143 223 d. Calculate the required rate of heat transfer to or from the reactor in kW. [30 Marks]Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


