Answered step by step
Verified Expert Solution
Question
1 Approved Answer
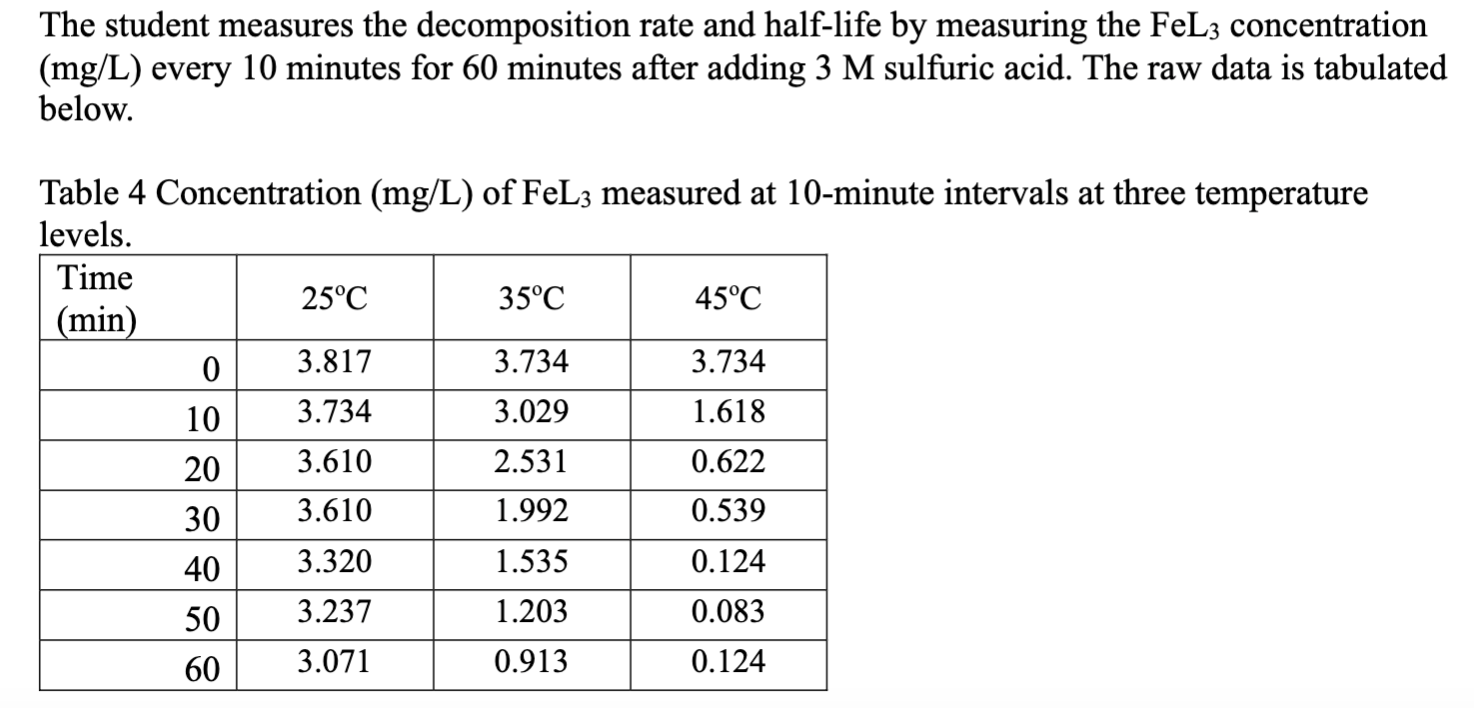
The student measures the decomposition rate and half-life by measuring the FeL3concentration (mg/L) every 10 minutes for 60 minutes after adding 3 M sulfuric acid.
The student measures the decomposition rate and half-life by measuring the FeL3concentration (mg/L) every 10 minutes for 60 minutes after adding 3 M sulfuric acid. The raw data is tabulated below. On a single graph, plot the natural logarithms of FeL3 concentration as a function of time for each temperature setting. Display linear regression equation and R 2 for each data set. Report the rate constants and half-life derived from these experiments.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


