Answered step by step
Verified Expert Solution
Question
1 Approved Answer
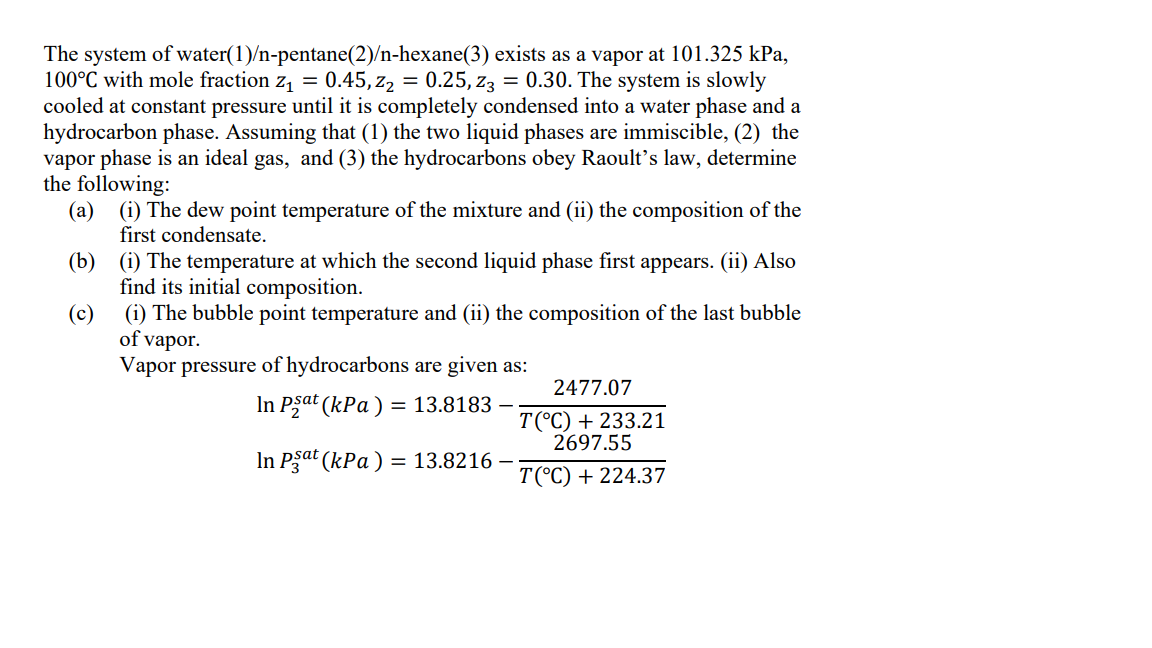
The system of water ( 1 ) / n - pentane ( 2 ) / n - hexane ( 3 ) exists as a vapor
The system of waternpentanenhexane exists as a vapor at kPa,
with mole fraction The system is slowly
cooled at constant pressure until it is completely condensed into a water phase and a
hydrocarbon phase. Assuming that the two liquid phases are immiscible, the
vapor phase is an ideal gas, and the hydrocarbons obey Raoult's law, determine
the following:
ai The dew point temperature of the mixture and ii the composition of the
first condensate.
bi The temperature at which the second liquid phase first appears. ii Also
find its initial composition.
ci The bubble point temperature and ii the composition of the last bubble
of vapor.
Vapor pressure of hydrocarbons are given as:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


