Question
The tetrahydropyran ring is a structural part of numerous antiviral drugs. Therefore, the development of efficient methods for the synthesis of this type of rings
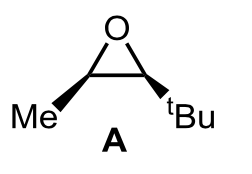
The tetrahydropyran ring is a structural part of numerous antiviral drugs. Therefore, the development of efficient methods for the synthesis of this type of rings is of great interest. To this must be added the importance of controlling the absolute configuration of the centers stereogenic properties of a drug by the impact on its activity. Below is a synthesis of the drug NTNPI-1127, an antiviral in the experimental phase: 3-(2-methoxyphenyl)-1-chloropropane was treated with magnesium in THF and the obtained organometallic derivative was made then react with oxirane A to isolate, diastereoselectively, a compound B after quenching the reaction with water. B was treated with bromine in the presence of light to obtain C(C17H27BrO2)) fully regioselectively as a 1:1 mixture of diastereoisomers. Next, C (the mixture of diastereoisomers) is dissolved in THF and treated with sodium hydride to obtain D (C17H2602) as a single diastereoisomer. Finally, treatment of compound D with HBr in refluxing toluene provided NTNPI-1127.
a) Represent the synthesis scheme developed for NTNPI-1127 indicating the structures of all the compounds, the mechanisms of the transformations involved and the selectivity of the reactions.
b) In the C->D transformation, a mixture of diastereoisomers of C is reacted obtaining D as a single pure stereoisomer. explain this fact
c) In another experiment, when oxirane A is treated with KOH, product E is isolated, whose treatment with concentrated sulfuric acid provides the ketone F selectively. explain this result.
d) On the other hand, compound B is treated with methanesulfonyl chloride to prepare G and a G is then subjected to reaction with NaOtBu obtaining H as a single diastereomer. Explain the diastereoselectivity obtained in the last step based on the transformation mechanism. Correctly represent the structure of all molecules.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


